Chapter 6 HW 2
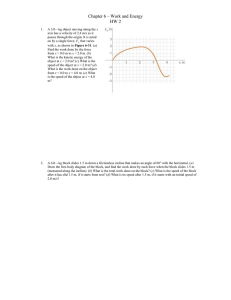
... A single horizontal force in the +x direction acts on a cart of mass m. The cart starts from rest at x = 0, and the speed of the cart increases with x as , where C is a constant. (a) Find the force acting on the cart as a function of x. (b) Find the work done by the force in moving the cart from x = ...
... A single horizontal force in the +x direction acts on a cart of mass m. The cart starts from rest at x = 0, and the speed of the cart increases with x as , where C is a constant. (a) Find the force acting on the cart as a function of x. (b) Find the work done by the force in moving the cart from x = ...
Concepts in Theoretical Physics
... Why do the quarks stick together in this way? It s because the quarks are the only particles to feel the strong nuclear force. To understand this better, we next need to look at the forces. ...
... Why do the quarks stick together in this way? It s because the quarks are the only particles to feel the strong nuclear force. To understand this better, we next need to look at the forces. ...
ParticleZoo
... The Quark Model The quark model represents a relatively simple picture of the internal structure of subatomic particles and makes predictions of their production and decay. It uses a minimum of adjusted quark parameters and has great predictive power, e.g., for the composite-particle masses, magnet ...
... The Quark Model The quark model represents a relatively simple picture of the internal structure of subatomic particles and makes predictions of their production and decay. It uses a minimum of adjusted quark parameters and has great predictive power, e.g., for the composite-particle masses, magnet ...
Lecture (2) - MIT OpenCourseWare
... backscatter (bounce back). This indicates that most of the mass of the atom is concentrated in a very small volume relative to the volume of the entire atom. We now call this the NUCLEUS. • Rutherford calculated the diameter of the nucleus to be _________ m. His calculation (see below) related the ...
... backscatter (bounce back). This indicates that most of the mass of the atom is concentrated in a very small volume relative to the volume of the entire atom. We now call this the NUCLEUS. • Rutherford calculated the diameter of the nucleus to be _________ m. His calculation (see below) related the ...
pages 1-2 of the lecture notes
... backscatter (bounce back). This indicates that most of the mass of the atom is concentrated in a very small volume relative to the volume of the entire atom. We now call this the NUCLEUS. • Rutherford calculated the diameter of the nucleus to be _________ m. His calculation (see below) related the ...
... backscatter (bounce back). This indicates that most of the mass of the atom is concentrated in a very small volume relative to the volume of the entire atom. We now call this the NUCLEUS. • Rutherford calculated the diameter of the nucleus to be _________ m. His calculation (see below) related the ...
Coulomb`s Law Handout
... - The nucleus is composed of two kinds of particles: protons and neutrons. - A proton has just about the same mass as a neutron. However, a proton is about 2000 times as massive as an electron. - Protons and electrons are attracted to one another. - Because of this force of attraction, elections "or ...
... - The nucleus is composed of two kinds of particles: protons and neutrons. - A proton has just about the same mass as a neutron. However, a proton is about 2000 times as massive as an electron. - Protons and electrons are attracted to one another. - Because of this force of attraction, elections "or ...
Exit Slip: Atomic Structure and Nuclear Chemistry-1
... transforms into a barium atom with 56 protons and the same number of total particles in its nucleus. Which kind of radiation is emitted by this decay (11.d)? A. x-ray C. gamma ray B. beta particle D. alpha particle ...
... transforms into a barium atom with 56 protons and the same number of total particles in its nucleus. Which kind of radiation is emitted by this decay (11.d)? A. x-ray C. gamma ray B. beta particle D. alpha particle ...
Forces of the Universe - The Federation of Galaxy Explorers
... in a force field describe how an object will move when placed near an object that emits a force. The Unified Theory For many years, scientists have been trying to find a way to tie the four forces together into one theory. This is called the ‘Unified’ theory. To date, there has been no way to tie th ...
... in a force field describe how an object will move when placed near an object that emits a force. The Unified Theory For many years, scientists have been trying to find a way to tie the four forces together into one theory. This is called the ‘Unified’ theory. To date, there has been no way to tie th ...
Ch 6 Work, Power, Energy
... 2 things are needed for there to be work ◦ Application of force ◦ Movement of object by force Forces parallel to direction of motion do work Forces perpendicular to direction of motion do no work ...
... 2 things are needed for there to be work ◦ Application of force ◦ Movement of object by force Forces parallel to direction of motion do work Forces perpendicular to direction of motion do no work ...
Pocket physics - National Physical Laboratory
... 1 volt is the potential difference (PD) between two points when 1 joule of electrical work is done per coulomb moving between those points. ...
... 1 volt is the potential difference (PD) between two points when 1 joule of electrical work is done per coulomb moving between those points. ...
Electrostatics Review Problems
... 2. How many protons must a compound have if it has 50 electrons and a net charge of +9.6 * 10-18 C? (110 protons) ...
... 2. How many protons must a compound have if it has 50 electrons and a net charge of +9.6 * 10-18 C? (110 protons) ...
KD3 Linear Mechanics
... 4 Types of Forces Cont.. • Strong Nuclear Force-Holds particles in the nucleus together • Weak Force-A form of electromagnetic force involved in the radio active decay of some nuclei ...
... 4 Types of Forces Cont.. • Strong Nuclear Force-Holds particles in the nucleus together • Weak Force-A form of electromagnetic force involved in the radio active decay of some nuclei ...
Exam.2
... The angle a is measured to be 18.0°. What is the speed of the mass? A) 1.95 m/s B) 3.82 m/s C) 1.18 m/s D) 1.09 m/s 7. An object is moving with constant velocity. Which of the following statements is true? A) A constant force is being applied in the direction of motion. B) There are no forces acting ...
... The angle a is measured to be 18.0°. What is the speed of the mass? A) 1.95 m/s B) 3.82 m/s C) 1.18 m/s D) 1.09 m/s 7. An object is moving with constant velocity. Which of the following statements is true? A) A constant force is being applied in the direction of motion. B) There are no forces acting ...
1. The frog leaps from its resting position at the lake`s bank onto a lily
... force of 14,000 N while accelerating at a rate of 8. (5 )What was the cars acceleration in the 5 m/s2? last 10 seconds? 0-15/10= -1.5 m/s2 a. 2800g b.2.8 x103Kg c.28 Kg d. 2.8x103 Kg 9. (5)Smooch had no seat belt so how much force did she travel through the window with? 5. Which answer does not rela ...
... force of 14,000 N while accelerating at a rate of 8. (5 )What was the cars acceleration in the 5 m/s2? last 10 seconds? 0-15/10= -1.5 m/s2 a. 2800g b.2.8 x103Kg c.28 Kg d. 2.8x103 Kg 9. (5)Smooch had no seat belt so how much force did she travel through the window with? 5. Which answer does not rela ...
Nuclear force

The nuclear force (or nucleon–nucleon interaction or residual strong force) is the force between protons and neutrons, subatomic particles that are collectively called nucleons. The nuclear force is responsible for binding protons and neutrons into atomic nuclei. Neutrons and protons are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience a Coulomb repulsion that tends to push them apart, but at short range the nuclear force is sufficiently attractive as to overcome the electromagnetic repulsive force. The mass of a nucleus is less than the sum total of the individual masses of the protons and neutrons which form it. The difference in mass between bound and unbound nucleons is known as the mass defect. Energy is released when nuclei break apart, and it is this energy that used in nuclear power and nuclear weapons.The nuclear force is powerfully attractive between nucleons at distances of about 1 femtometer (fm, or 1.0 × 10−15 metres) between their centers, but rapidly decreases to insignificance at distances beyond about 2.5 fm. At distances less than 0.7 fm, the nuclear force becomes repulsive. This repulsive component is responsible for the physical size of nuclei, since the nucleons can come no closer than the force allows. By comparison, the size of an atom, measured in angstroms (Å, or 1.0 × 10−10 m), is five orders of magnitude larger. The nuclear force is not simple, however, since it depends on the nucleon spins, has a tensor component, and may depend on the relative momentum of the nucleons.A quantitative description of the nuclear force relies on partially empirical equations that model the internucleon potential energies, or potentials. (Generally, forces within a system of particles can be more simply modeled by describing the system's potential energy; the negative gradient of a potential is equal to the vector force.) The constants for the equations are phenomenological, that is, determined by fitting the equations to experimental data. The internucleon potentials attempt to describe the properties of nucleon–nucleon interaction. Once determined, any given potential can be used in, e.g., the Schrödinger equation to determine the quantum mechanical properties of the nucleon system.The discovery of the neutron in 1932 revealed that atomic nuclei were made of protons and neutrons, held together by an attractive force. By 1935 the nuclear force was conceived to be transmitted by particles called mesons. This theoretical development included a description of the Yukawa potential, an early example of a nuclear potential. Mesons, predicted by theory, were discovered experimentally in 1947. By the 1970s, the quark model had been developed, which showed that the mesons and nucleons were composed of quarks and gluons. By this new model, the nuclear force, resulting from the exchange of mesons between neighboring nucleons, is a residual effect of the strong force.