Microbiology bio 123
... Metabolism includes two major types of reactions: 1. Endergonic reactions require energy to be placed into the reaction to complete the metabolism; typically biosynthetic reactions. Referred to as anabolism. 2. Exergonic reactions produce energy when the reaction takes place in the form of ATP. Typi ...
... Metabolism includes two major types of reactions: 1. Endergonic reactions require energy to be placed into the reaction to complete the metabolism; typically biosynthetic reactions. Referred to as anabolism. 2. Exergonic reactions produce energy when the reaction takes place in the form of ATP. Typi ...
CH 2. CELLULAR RESPIRATION
... respiration is as follows: C6H12O6(aq) + 6O2(g) 6CO2(g) + 6H2O(l) + 36 ATP glucose ...
... respiration is as follows: C6H12O6(aq) + 6O2(g) 6CO2(g) + 6H2O(l) + 36 ATP glucose ...
video slide
... pH gradient ATP About 40% of the energy is transferred to ATP -making about 38 ATP ...
... pH gradient ATP About 40% of the energy is transferred to ATP -making about 38 ATP ...
2 H
... • Aerobic: – Absolute need of oxygen to survive – Used as a final electron acceptor – Used by bacteria that carry out an oxidative or aerobic respiratory metabolism ...
... • Aerobic: – Absolute need of oxygen to survive – Used as a final electron acceptor – Used by bacteria that carry out an oxidative or aerobic respiratory metabolism ...
Chapter 7 How Cells Release Chemical energy
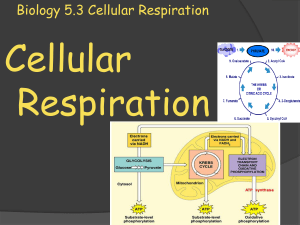
... Glycolysis – Glucose Breakdown Starts First step – Conversion of glucose to pyruvate Enzymes of glycolysis use two ATP to convert one molecule of glucose to two molecules of three-carbon pyruvate Reactions transfer electrons and hydrogen atoms to two NAD+ (reduces to NADH) 2 ATP is formed by substr ...
... Glycolysis – Glucose Breakdown Starts First step – Conversion of glucose to pyruvate Enzymes of glycolysis use two ATP to convert one molecule of glucose to two molecules of three-carbon pyruvate Reactions transfer electrons and hydrogen atoms to two NAD+ (reduces to NADH) 2 ATP is formed by substr ...
The Structure and Hydrolysis of ATP
... • In cellular respiration, glucose and other organic molecules are broken down in a series of steps • Electrons from organic compounds are usually first transferred to NAD+, a coenzyme • As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration • Each NADH (the re ...
... • In cellular respiration, glucose and other organic molecules are broken down in a series of steps • Electrons from organic compounds are usually first transferred to NAD+, a coenzyme • As an electron acceptor, NAD+ functions as an oxidizing agent during cellular respiration • Each NADH (the re ...
Guided reading Ch 9- ENERGY IN A CELL
... Because of faulty instructions in her DNA, one of the proteins in her ETC is misshapen, and therefore it cannot perform its function as efficiently. Baby Helen is “failing to thrive” as she is having a hard time gaining weight and reaching developmental milestones. Using your knowledge of the ETC an ...
... Because of faulty instructions in her DNA, one of the proteins in her ETC is misshapen, and therefore it cannot perform its function as efficiently. Baby Helen is “failing to thrive” as she is having a hard time gaining weight and reaching developmental milestones. Using your knowledge of the ETC an ...
Cell Respiration powerpoint slides
... compound, thus forming citric acid. As the citric acid proceeds through the cycle, it is first broken down into a 5-carbon compound, and then into a 4-carbon ...
... compound, thus forming citric acid. As the citric acid proceeds through the cycle, it is first broken down into a 5-carbon compound, and then into a 4-carbon ...
chapter 11 - rci.rutgers.edu
... contrast to glycolysis which is anaerobic. The CAC takes place in the mitochondrial matrix of eukaryotic cells – whereas glycolysis occurs in the cytoplasm. The immediate products of the CAC are reduced cofactors (NADH and FADH2) which then feed electrons into oxidative phosphorylation, yielding muc ...
... contrast to glycolysis which is anaerobic. The CAC takes place in the mitochondrial matrix of eukaryotic cells – whereas glycolysis occurs in the cytoplasm. The immediate products of the CAC are reduced cofactors (NADH and FADH2) which then feed electrons into oxidative phosphorylation, yielding muc ...
File - Mr. Shanks` Class
... a) Glucose – 6 – phosphate b) Fructose – 6 phosphate c) Fructose - 1 ,6 biphosphate d) Glucose 3. Which one of the following is a reduced electron carrier that carries electrons to the ETC? a) GDP b) NADH+H+ c) NAD d) ADP 4. What are the names of both 3 carbon molecules that result when glucose is b ...
... a) Glucose – 6 – phosphate b) Fructose – 6 phosphate c) Fructose - 1 ,6 biphosphate d) Glucose 3. Which one of the following is a reduced electron carrier that carries electrons to the ETC? a) GDP b) NADH+H+ c) NAD d) ADP 4. What are the names of both 3 carbon molecules that result when glucose is b ...
Energy and Respiration
... At high carbon dioxide concentrations as are found at the cell level hemoglobin binds to the carbon dioxide molecules which are then transported back to the lungs where the carbon dioxide is released and exhaled. ...
... At high carbon dioxide concentrations as are found at the cell level hemoglobin binds to the carbon dioxide molecules which are then transported back to the lungs where the carbon dioxide is released and exhaled. ...
Ch 9 Cellular respiration
... mostly made of proteins multiprotein complexes #IIV prosthetic gps. are attached to these proteins nonprotein needed for catalysis by enzymes during chainelectron carriers alternate between reduced and oxidized states as they gain and lose electrons ...
... mostly made of proteins multiprotein complexes #IIV prosthetic gps. are attached to these proteins nonprotein needed for catalysis by enzymes during chainelectron carriers alternate between reduced and oxidized states as they gain and lose electrons ...
Cell Respiration RG
... 3. Why is being “reduced” equivalent to having a greater potential energy? ...
... 3. Why is being “reduced” equivalent to having a greater potential energy? ...
Cellular Respiration
... Except for muscles which can get rid of their H atoms during glycolysis because the H are passed back to pyruvate and lactic acid is formed. ...
... Except for muscles which can get rid of their H atoms during glycolysis because the H are passed back to pyruvate and lactic acid is formed. ...
Cellular Respiration
... • The mitochondria are the engines of our cells where sugar is burned for fuel and the exhaust is CO2 and H2O. ...
... • The mitochondria are the engines of our cells where sugar is burned for fuel and the exhaust is CO2 and H2O. ...
Exam 1 Q2 Review Sheet
... used/made and NADH/FADH2 made for every step. (The following terms MUST be properly included: ETC, chemiosmosis, oxidative phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokina ...
... used/made and NADH/FADH2 made for every step. (The following terms MUST be properly included: ETC, chemiosmosis, oxidative phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokina ...
Biochemistry 2
... B. They are usually coupled with anabolic pathways to which they supply energy in the form of ATP C. They involve endergonic reactions that break complex molecules into simpler ones D. They do not need enzyme catalysts E. They build up complex molecules such as protein from simpler compounds. ...
... B. They are usually coupled with anabolic pathways to which they supply energy in the form of ATP C. They involve endergonic reactions that break complex molecules into simpler ones D. They do not need enzyme catalysts E. They build up complex molecules such as protein from simpler compounds. ...
PP Chapter 9 - WordPress.com
... • Electrons are transferred from NADH or FADH2 to the electron transport chain ...
... • Electrons are transferred from NADH or FADH2 to the electron transport chain ...
PP Chapter 9 - Trimble County Schools
... • Electrons are transferred from NADH or FADH2 to the electron transport chain ...
... • Electrons are transferred from NADH or FADH2 to the electron transport chain ...
Electron transport chain
An electron transport chain (ETC) is a series of compounds that transfer electrons from electron donors to electron acceptors via redox reactions, and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. This creates an electrochemical proton gradient that drives ATP synthesis, or the generation of chemical energy in the form of adenosine triphosphate (ATP). The final acceptor of electrons in the electron transport chain is molecular oxygen.Electron transport chains are used for extracting energy via redox reactions from sunlight in photosynthesis or, such as in the case of the oxidation of sugars, cellular respiration. In eukaryotes, an important electron transport chain is found in the inner mitochondrial membrane where it serves as the site of oxidative phosphorylation through the use of ATP synthase. It is also found in the thylakoid membrane of the chloroplast in photosynthetic eukaryotes. In bacteria, the electron transport chain is located in their cell membrane.In chloroplasts, light drives the conversion of water to oxygen and NADP+ to NADPH with transfer of H+ ions across chloroplast membranes. In mitochondria, it is the conversion of oxygen to water, NADH to NAD+ and succinate to fumarate that are required to generate the proton gradient. Electron transport chains are major sites of premature electron leakage to oxygen, generating superoxide and potentially resulting in increased oxidative stress.