11129_evl_ch1_ste_corr
... electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
... electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
CCH 3 Mole Notes
... Atoms of a given element are identical in size, mass and other properties (Not true today, since Dalton we have discovered isotopes. Isotopes are atoms of the same element that differ in number of neutrons and atomic mass KNOW-(Hydrogen has 3 isotopes, protium 11H (1 proton and 0 neutrons), deuteriu ...
... Atoms of a given element are identical in size, mass and other properties (Not true today, since Dalton we have discovered isotopes. Isotopes are atoms of the same element that differ in number of neutrons and atomic mass KNOW-(Hydrogen has 3 isotopes, protium 11H (1 proton and 0 neutrons), deuteriu ...
chemistry chapter 5 notes
... Rutherford’s model of the atom stated that the atom is mostly empty space with all the positive charge and almost all of the mass concentrated in a small region, which he called the nucleus. ...
... Rutherford’s model of the atom stated that the atom is mostly empty space with all the positive charge and almost all of the mass concentrated in a small region, which he called the nucleus. ...
1.3 UNDERSTANDING ATOMIC MASS
... 3. The periodic law came to be accepted because of Mendeleev’s detailed predictions of the properties of undiscovered elements, using his knowledge of periodic trends. 4. (a) liquids at SATP: bromine, mercury; gases at SATP: hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypto ...
... 3. The periodic law came to be accepted because of Mendeleev’s detailed predictions of the properties of undiscovered elements, using his knowledge of periodic trends. 4. (a) liquids at SATP: bromine, mercury; gases at SATP: hydrogen, helium, nitrogen, oxygen, fluorine, neon, chlorine, argon, krypto ...
Physical Science
... Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy l ...
... Each row in the table of elements is a period. • Hydrogen, the first element in Period 1, has one electron in its first energy level. • Lithium, the first element in Period 2, has one electron in its second energy level. • Sodium, the first element in Period 3, has one electron in its third energy l ...
GTthe_atom - Science
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...
View/Open - Rice Scholarship Home
... number of atoms all having the same chemical properties, and, therefore, all having the same nuclear charges and thg same number of electrons when in the neutral state. I t has usually been assumed that all the atoms of a chemical element have equal weights, but we see now that this may not be true, ...
... number of atoms all having the same chemical properties, and, therefore, all having the same nuclear charges and thg same number of electrons when in the neutral state. I t has usually been assumed that all the atoms of a chemical element have equal weights, but we see now that this may not be true, ...
Radioactivity
... best. Light elements tend to have about as many neutrons as protons; heavy elements apparently need more neutrons than protons in order to stick together. Atoms with a few too many neutrons, or not quite enough, can sometimes exist for a while, but they're unstable. Unstable atoms are radioactive: t ...
... best. Light elements tend to have about as many neutrons as protons; heavy elements apparently need more neutrons than protons in order to stick together. Atoms with a few too many neutrons, or not quite enough, can sometimes exist for a while, but they're unstable. Unstable atoms are radioactive: t ...
The Atom
... what is the charge of the resulting ion? 2) How many electrons would be found in the ion O2-? 3) If an ion has 28 protons and 26 electrons, what is its charge? What is its symbol (including charge)? ...
... what is the charge of the resulting ion? 2) How many electrons would be found in the ion O2-? 3) If an ion has 28 protons and 26 electrons, what is its charge? What is its symbol (including charge)? ...
IX Chemistry Chapter 03
... 3.5. ELECTRONIC CONFIGURATION BASED ON BOHR'S MODEL When atoms react, it is actually the electrons that interact. For this reason, the arrangement of electrons is responsible for the structure of atom. The arrangement of electrons refers not only to the number of electrons that an atom possesses, bu ...
... 3.5. ELECTRONIC CONFIGURATION BASED ON BOHR'S MODEL When atoms react, it is actually the electrons that interact. For this reason, the arrangement of electrons is responsible for the structure of atom. The arrangement of electrons refers not only to the number of electrons that an atom possesses, bu ...
CP Chemistry Atomic Structure TEST 1. The Greek philosopher
... D. none of the above 24. The current periodic table is arranged in order of A. atomic number B. alphabetical symbols ...
... D. none of the above 24. The current periodic table is arranged in order of A. atomic number B. alphabetical symbols ...
Henry Moseley, the Atomic Number, and Synthesis
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
Henry Moseley, the Atomic Number, and Synthesis
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
... to advance the understanding of the elements and solve the problem with Mendeleev’s periodic table. Explain that organizing the elements by their weight did not always give a periodic alignment of their chemical properties. Moseley noticed that shooting electrons at elements caused them to release x ...
11129_evl_ch1_ste_eleve (3)
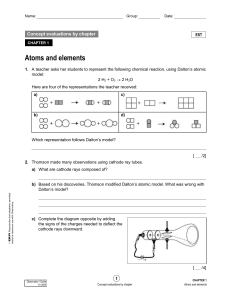
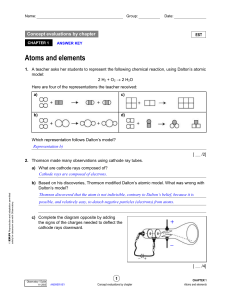
... 7. Six different elements are represented below according to the Rutherford-Bohr atomic model. ...
... 7. Six different elements are represented below according to the Rutherford-Bohr atomic model. ...
Atoms HW 1/31 - Westerville City Schools
... electron. Compared with the protons and neutrons, the electron is extremely light coming in almost 2000 times lighter than a proton. For this reason, the mass of an atom listed on the periodic table ignores the weight of an electron. So even though the electron takes up most of the space of an atom ...
... electron. Compared with the protons and neutrons, the electron is extremely light coming in almost 2000 times lighter than a proton. For this reason, the mass of an atom listed on the periodic table ignores the weight of an electron. So even though the electron takes up most of the space of an atom ...
Chem 115 POGIL Worksheet - Week 10 Periodic Trends Why? The
... Owing to their relatively low ionization energies, metals tend to form cations, and when they combine with nonmetals they form ionic substances. For example, when metals combine with oxygen they form ionic oxides. 4 Fe(s) + 3 O2 2 Fe2O3(s) Metal oxides tend to dissolve in water to form hydroxide i ...
... Owing to their relatively low ionization energies, metals tend to form cations, and when they combine with nonmetals they form ionic substances. For example, when metals combine with oxygen they form ionic oxides. 4 Fe(s) + 3 O2 2 Fe2O3(s) Metal oxides tend to dissolve in water to form hydroxide i ...
Chapter 4, Lesson 2: The Periodic Table
... Each student should find and present some basic information about their element to the class. The presentation can be in the form of a poster, pamphlet, PowerPoint presentation or other form. The presentations should be short and can include: atom name, atomic number, derivation of name, when and wh ...
... Each student should find and present some basic information about their element to the class. The presentation can be in the form of a poster, pamphlet, PowerPoint presentation or other form. The presentations should be short and can include: atom name, atomic number, derivation of name, when and wh ...
Chapter 2: Atoms Molecules and Ions
... reactant molecules into a mass ratio for a chemical reaction to be useful. 2) Mass ratios are determined by using atomic masses for the elements. i) Atomic masses (atomic weights) are found in periodic table beneath the chemical symbol, and represent the average of all the naturally occurring isotop ...
... reactant molecules into a mass ratio for a chemical reaction to be useful. 2) Mass ratios are determined by using atomic masses for the elements. i) Atomic masses (atomic weights) are found in periodic table beneath the chemical symbol, and represent the average of all the naturally occurring isotop ...
5Periodic Table of Elements WB
... group his cards according to team or position. Biologists classify all living organisms in a fivekingdom classification system, based on similar characteristics. In 1869 Dmitri Mendeleev, a Russian Chemist, published the first periodic table. It had eight columns and it contained blank spaces for el ...
... group his cards according to team or position. Biologists classify all living organisms in a fivekingdom classification system, based on similar characteristics. In 1869 Dmitri Mendeleev, a Russian Chemist, published the first periodic table. It had eight columns and it contained blank spaces for el ...
- Science
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...
... The term matter describes all of the physical substances around us: your table, your body, a pencil, water, and so forth ...