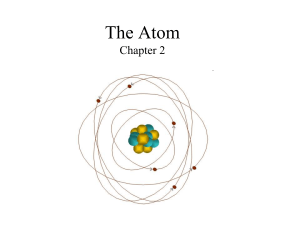
Atomic Mass
... Atomic masses can be different for atoms of the same element if they have different numbers of neutrons Atoms with different masses are called Isotopes or Nuclides ...
... Atomic masses can be different for atoms of the same element if they have different numbers of neutrons Atoms with different masses are called Isotopes or Nuclides ...
key - Greenslime.info
... group? The number of valence electrons, and thus the chemical properties. ...
... group? The number of valence electrons, and thus the chemical properties. ...
PP - myndrs.com
... • How do we find the number of Neutrons? (mass number) - (atomic number) =# of neutrons ...
... • How do we find the number of Neutrons? (mass number) - (atomic number) =# of neutrons ...
Structure of an Atom structure_of_atom
... • How do we find the number of Neutrons? (mass number) - (atomic number) =# of neutrons ...
... • How do we find the number of Neutrons? (mass number) - (atomic number) =# of neutrons ...
The Atom Chapter 2
... Hund’s Rule: electrons pair only after each orbital of equal energy is occupied by a single electron ...
... Hund’s Rule: electrons pair only after each orbital of equal energy is occupied by a single electron ...
Atom through Periodic Table Study Guide
... 16. Periods go ____(which way) on the periodic table, while rows go ____(which way). 17. Which family has elements that are highly reactive non-metals and has 7 valence electrons? 18. The noble gases are found in which column of the periodic table? 19. The noble gases are non-reactive- they won’t b ...
... 16. Periods go ____(which way) on the periodic table, while rows go ____(which way). 17. Which family has elements that are highly reactive non-metals and has 7 valence electrons? 18. The noble gases are found in which column of the periodic table? 19. The noble gases are non-reactive- they won’t b ...
Practice Test #2 - smhs
... Ag-109 has a mass of 108.9047 amu. The periodic table gives the atomic weight for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. ...
... Ag-109 has a mass of 108.9047 amu. The periodic table gives the atomic weight for Ag as 107.868 amu. Find the percent abundance of the lighter isotope of Ag. ...
Atomic structure and periodic table
... Build up of electrons in shells The horizontal rows are called periods (numbered from 1-7) Each period represents the filling of one electron shell Shell number 1 can contain only 2 electrons, therefore period one has only 2 elements, hydrogen and helium. Shell number 2 can contain 8 electro ...
... Build up of electrons in shells The horizontal rows are called periods (numbered from 1-7) Each period represents the filling of one electron shell Shell number 1 can contain only 2 electrons, therefore period one has only 2 elements, hydrogen and helium. Shell number 2 can contain 8 electro ...
1000 - Paint Valley Local Schools
... What is form compounds? The alkali metals, found in group 1 of the periodic table are metals that do not occur freely in nature. These metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. Thi ...
... What is form compounds? The alkali metals, found in group 1 of the periodic table are metals that do not occur freely in nature. These metals have only one valence electron in their outer shell. Therefore, they are ready to lose that one electron in ionic bonding with other elements very easily. Thi ...
Thursday, October 31, 2013 D-day
... • Radioactive Elements- no naturally occurring stable isotope (what is an isotope?). – These elements loose neutrons and protons and emit them as particles. – All manmade elements are radioactive. ...
... • Radioactive Elements- no naturally occurring stable isotope (what is an isotope?). – These elements loose neutrons and protons and emit them as particles. – All manmade elements are radioactive. ...
Part A: Multiple Choice. Circle the letter
... 7. In general, which of the following properties does NOT increase across a row from left to right? a) atomic number b) atomic radius c) nuclear charge d) ionization energy e) electron affinity 8. Which of the following properties decreases from top to bottom in a column? a) ionization energy b) ato ...
... 7. In general, which of the following properties does NOT increase across a row from left to right? a) atomic number b) atomic radius c) nuclear charge d) ionization energy e) electron affinity 8. Which of the following properties decreases from top to bottom in a column? a) ionization energy b) ato ...
Atomic Structure
... ________ The Alkali Earth with the smallest atomic radius. ________ Element number 14 (element with atomic number 14). ________ The second period noble gas. ________ The first metal in group 1. ________ An element that reacts like chlorine, but has a smaller atomic radius. ________ A third period el ...
... ________ The Alkali Earth with the smallest atomic radius. ________ Element number 14 (element with atomic number 14). ________ The second period noble gas. ________ The first metal in group 1. ________ An element that reacts like chlorine, but has a smaller atomic radius. ________ A third period el ...
2 IONS
... #1 ATOMIC SIZE – decreases across and increases down the periodic table #2 IONSLeft side of the periodic table forms positive ions. Group 1- charge of +1 (lose an electron) Group 2- charge of +2 (lose 2 electrons) Groups 3-12 (form positive ions with varying charges) Group 17-charge of -1 (gain an ...
... #1 ATOMIC SIZE – decreases across and increases down the periodic table #2 IONSLeft side of the periodic table forms positive ions. Group 1- charge of +1 (lose an electron) Group 2- charge of +2 (lose 2 electrons) Groups 3-12 (form positive ions with varying charges) Group 17-charge of -1 (gain an ...
No Slide Title - Mercer Island School District
... Tin Use both long hand and nobel gas configuration ...
... Tin Use both long hand and nobel gas configuration ...
The Nature of Molecules
... • Diagram of typical atomic structure: • Atomic #/mass of: H, He, C, O, N, S, P, Ne ...
... • Diagram of typical atomic structure: • Atomic #/mass of: H, He, C, O, N, S, P, Ne ...
Chemical Change
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
... The chemical properties of elements are related to the energy changes that take place when atoms lose, gain or share electrons to obtain a filled valence shell. ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... become more stable by emitting radiation. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. This radiation can be emitted in the form of a positively charged alpha particle, a negatively charged ...
... become more stable by emitting radiation. Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. This radiation can be emitted in the form of a positively charged alpha particle, a negatively charged ...
Biol 1441
... Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ion Anion: a negative ion The transfer of an electron is not the formation of a bond; rather ...
... Two atoms are so unequal in their attraction for valence electrons that the more electronegative atom strips an electron completely away from its partner. Ion: a charged atom (or molecule) Cation: a positive ion Anion: a negative ion The transfer of an electron is not the formation of a bond; rather ...
Test 1
... The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an element contain different numbers of neutrons. The three pr ...
... The mass of an atom in amu is approximated as the number of photons plus the number of neutrons present in the nucleus. Atoms can be split into a nucleus and the electrons, and the electrons move around the nucleus. Different isotopes of an element contain different numbers of neutrons. The three pr ...
The Chemical Context of Life
... The different stages of energy that electrons have are called energy levels, or electron shells ...
... The different stages of energy that electrons have are called energy levels, or electron shells ...
File
... 6. What properties to metals, nonmetals, and metalloids have? Metals - Shiny luster, malleable, some are magnetic, good conductors of electricity and heat. Nonmetals – dull luster, brittle, nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
... 6. What properties to metals, nonmetals, and metalloids have? Metals - Shiny luster, malleable, some are magnetic, good conductors of electricity and heat. Nonmetals – dull luster, brittle, nonmagnetic, insulators. Metalloids- properties of both, sometimes called semi-conductors. ...
Radioisotopes
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...
... having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number of nucleons, the number of protons plus neutron ...