Atomic Structure 1
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
Elements Unit Test
... 9. A Dutch scientist by the name of Neils Bohr was the first scientist to figure out that electrons that orbit the nucleus of an atom (sometimes called shells) can only hold a certain number of electrons. Which of the following sets of numbers describe the number of electrons in the first three shel ...
... 9. A Dutch scientist by the name of Neils Bohr was the first scientist to figure out that electrons that orbit the nucleus of an atom (sometimes called shells) can only hold a certain number of electrons. Which of the following sets of numbers describe the number of electrons in the first three shel ...
Honors Review Unit 2 answers
... 15. Write the symbols for an alpha particle, beta particle, and gamma ray. Alpha particle – 42He; ...
... 15. Write the symbols for an alpha particle, beta particle, and gamma ray. Alpha particle – 42He; ...
Notes-Periodic Table (2nd Part)
... Reactivity in Elements For groups 1 to 2: Reactivity increases from right to left For groups 13 to 17: (ignore group 18…they are non-reactive!) Reactivity increases from left to right Groups 1 to 2 ...
... Reactivity in Elements For groups 1 to 2: Reactivity increases from right to left For groups 13 to 17: (ignore group 18…they are non-reactive!) Reactivity increases from left to right Groups 1 to 2 ...
electrons - Northside Middle School
... sooooo reactive. They all have really empty valence shells. They each only have one electron in their valence shell! Nobel gases are not reactive because they have full valence shells! ...
... sooooo reactive. They all have really empty valence shells. They each only have one electron in their valence shell! Nobel gases are not reactive because they have full valence shells! ...
Section 2A
... Origins of the Periodic Table In 1869, approximately 62 elements were known to exist. Scientists wanted a convenient way to look at these elements. Dmitri Mendeleev proposed a periodic table of elements. It was arranged by increasing mass number and similar properties. Later, Henry Mosley working wi ...
... Origins of the Periodic Table In 1869, approximately 62 elements were known to exist. Scientists wanted a convenient way to look at these elements. Dmitri Mendeleev proposed a periodic table of elements. It was arranged by increasing mass number and similar properties. Later, Henry Mosley working wi ...
ISOTOPES 3 SUBATOMIC PARTICLES Proton Located inside the
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
Atoms and Elements - Dublin City Schools
... • For our purposes, there are four rings around the nucleus. • The closest ring holds 2 electrons. • The second ring holds 8 electrons. • The third ring holds 8 electrons. • The fourth ring holds 2 electrons. ...
... • For our purposes, there are four rings around the nucleus. • The closest ring holds 2 electrons. • The second ring holds 8 electrons. • The third ring holds 8 electrons. • The fourth ring holds 2 electrons. ...
Atomic Number
... -Elements within the same group have similar properties EX. Au, Ag, Cu -Each horizontal row is called a ____________________ -Properties of the elements gradually change when you move through a period -Elements get smaller when you move from _________________ to ______________. ...
... -Elements within the same group have similar properties EX. Au, Ag, Cu -Each horizontal row is called a ____________________ -Properties of the elements gradually change when you move through a period -Elements get smaller when you move from _________________ to ______________. ...
Chemistry Test Review - Greenslime Home Page
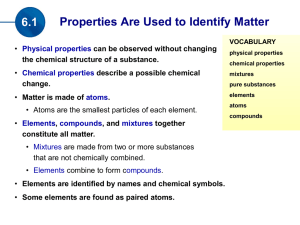
... a. Atom – the smallest part of an element that still acts like that element; can’t be broken down; basic part of matter b. Element – a substance that cannot be broken down into simpler substances containing only 1 type of atom c. Compound – two or more different elements chemically combined d. Molec ...
... a. Atom – the smallest part of an element that still acts like that element; can’t be broken down; basic part of matter b. Element – a substance that cannot be broken down into simpler substances containing only 1 type of atom c. Compound – two or more different elements chemically combined d. Molec ...
Early History of Atomic Theories
... Because of their electrons… All atoms of same ELEMENT have: unique # protons …THEREFORE… unique # electrons ...
... Because of their electrons… All atoms of same ELEMENT have: unique # protons …THEREFORE… unique # electrons ...
Atoms have a structure that determines their properties.
... • Metals are found on the left side, non-metals on the right, and metalloids in between. • Chemical families are arranged in vertical groups. • The table can also display the chemical symbol, atomic number, atomic mass, ion charge, density, and other information about each element. ...
... • Metals are found on the left side, non-metals on the right, and metalloids in between. • Chemical families are arranged in vertical groups. • The table can also display the chemical symbol, atomic number, atomic mass, ion charge, density, and other information about each element. ...
Periodic Table Review Key
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
Periodic Table Quiz
... 11. Which element is in Period 4, Group 13? a) Na b) Al c) Ga d) K 12. How many electrons does Sulfur contain? a) 15 b) 16 c) 17 d) 18 13. Which elements below are ALL nonmetals? a) K, Ca, Sc b) Be, Br, Kr c) V, Cr, Mn d) Ne, Cl, Br ...
... 11. Which element is in Period 4, Group 13? a) Na b) Al c) Ga d) K 12. How many electrons does Sulfur contain? a) 15 b) 16 c) 17 d) 18 13. Which elements below are ALL nonmetals? a) K, Ca, Sc b) Be, Br, Kr c) V, Cr, Mn d) Ne, Cl, Br ...
ATOMS AND ELEMENTS
... A. Electrons travel around the nucleus in the electron cloud. B. Electrons follow paths called energy levels or energy shells. C. All elements have at least 1 energy level. D. The period number (or the rows) on the Periodic Table tells you the number of occupied energy shells that element has. E. El ...
... A. Electrons travel around the nucleus in the electron cloud. B. Electrons follow paths called energy levels or energy shells. C. All elements have at least 1 energy level. D. The period number (or the rows) on the Periodic Table tells you the number of occupied energy shells that element has. E. El ...
Unit 4 – Atomic Structure Study Guide
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
... Dalton considered atoms to be whole and indivisible, that is, only whole atoms can be combined to form compounds. In the above formula, there are 1.5 Mg atoms, which is not possible based upon the indivisibility of an atom. 4. Complete the following table on the subatomic particles. PARTICLE Proto ...
Chemistry Midterm Exam 2015 (Study Guide) Unit 1: Measurement
... 21. What is the density of an object having a mass of 8.0 g and a volume of 25 cm ? 8/25=0.32g/cm3 Unit 2: Atomic History & Structure Define: ...
... 21. What is the density of an object having a mass of 8.0 g and a volume of 25 cm ? 8/25=0.32g/cm3 Unit 2: Atomic History & Structure Define: ...
levels of organization and the atom
... The # of protons determines what type of element is that particular atom. The ATOMIC ENERGY comes from the nucleus. ...
... The # of protons determines what type of element is that particular atom. The ATOMIC ENERGY comes from the nucleus. ...
Standard Atomic Notation 17 35 mass # atomic
... All matter is made of tiny particles Each element has its own kind of atom and mass Compounds are created when elements ...
... All matter is made of tiny particles Each element has its own kind of atom and mass Compounds are created when elements ...
Test 2 Review Test 2 Review (15-16)_2
... (14) ____________ Which column from above contains the alkaline earth metals? (15) ____________ Which column from above contains the Noble Gases? (16) ____________ Which column from above contains the most reactive non-metals? (17) ____________ Which column from above contains VERY non-reactive elem ...
... (14) ____________ Which column from above contains the alkaline earth metals? (15) ____________ Which column from above contains the Noble Gases? (16) ____________ Which column from above contains the most reactive non-metals? (17) ____________ Which column from above contains VERY non-reactive elem ...
What is the smallest particle of an element that retains the properties
... 3. T or F. Electrons are positively charged and are the lightest of the three subatomic particles. 4. Which subatomic particle has a +1 charge? Which has a 0 charge? 5. In Rutherford’s gold foil experiment, most of the positively charged particles which he shot at the foil ________________, while a ...
... 3. T or F. Electrons are positively charged and are the lightest of the three subatomic particles. 4. Which subatomic particle has a +1 charge? Which has a 0 charge? 5. In Rutherford’s gold foil experiment, most of the positively charged particles which he shot at the foil ________________, while a ...
Periodic Table Vocab page 7
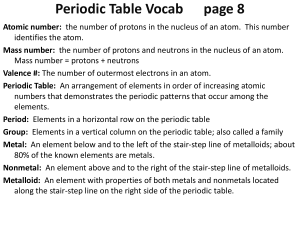
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
notes 4.1 & 4.2
... Do we have to know this? • But you’ll hear more about that in chemistry. For now realize that each energy level is made up of sublevels that hold specific amounts of electrons. • The sublevels are called orbitals and are named s, p, d, f • You can see these patterns in the periodic table. ...
... Do we have to know this? • But you’ll hear more about that in chemistry. For now realize that each energy level is made up of sublevels that hold specific amounts of electrons. • The sublevels are called orbitals and are named s, p, d, f • You can see these patterns in the periodic table. ...
Elements and Atoms
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...
... • To make molecules, you must have elements. • Elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. ...