Atomic Physics
... Rutherford’s model does not predict the existence of discrete energy levels in atoms. ...
... Rutherford’s model does not predict the existence of discrete energy levels in atoms. ...
Slide 1
... • Neutrons and protons have almost the same mass • Atoms always have as many electrons as protons • Atoms usually have about as many neutrons as protons e.g. Hydrogen (1 proton, 1 electron, 0 neutron) ; Helium (2 proton, 2 electron, 2 neutron) ; Carbon ( 6 proton, 6 electron, 6 neutron). • Add a pro ...
... • Neutrons and protons have almost the same mass • Atoms always have as many electrons as protons • Atoms usually have about as many neutrons as protons e.g. Hydrogen (1 proton, 1 electron, 0 neutron) ; Helium (2 proton, 2 electron, 2 neutron) ; Carbon ( 6 proton, 6 electron, 6 neutron). • Add a pro ...
Prerequisites Level Year Number of Study Hours Course Code
... bremsstrahlung, Photon interaction with matter, Photoelectric, Compton and pair production mechanism, Interaction of neutrons with matter, Interaction of hadrons with matter (briefly). Particle Detection: Ionization detectors, Ionization counters, proportional counters and Geiger Muller counters, Sc ...
... bremsstrahlung, Photon interaction with matter, Photoelectric, Compton and pair production mechanism, Interaction of neutrons with matter, Interaction of hadrons with matter (briefly). Particle Detection: Ionization detectors, Ionization counters, proportional counters and Geiger Muller counters, Sc ...
Concepts of Modern Physics Presentations
... SPH4U: Concepts of Modern Physics Presentations Guidelines: You will choose a topic in modern physics from the list below. Each topic is a basic concept or idea in modern physics (related to quantum mechanics, general relativity, particle physics, or theories of everything) You will be allotted 10 m ...
... SPH4U: Concepts of Modern Physics Presentations Guidelines: You will choose a topic in modern physics from the list below. Each topic is a basic concept or idea in modern physics (related to quantum mechanics, general relativity, particle physics, or theories of everything) You will be allotted 10 m ...
Lecture 2
... produces additional magnetic effects. So far as electrical effects are concerned, objects can be electrically neutral, positively charged, or negatively charged. Positively charged particles, such as the protons that are found in the nucleus of atoms, repel one another. Negatively charged particles, ...
... produces additional magnetic effects. So far as electrical effects are concerned, objects can be electrically neutral, positively charged, or negatively charged. Positively charged particles, such as the protons that are found in the nucleus of atoms, repel one another. Negatively charged particles, ...
explanation
... of 3.4 eV (first excited level) and so on. As the quantum number n increases the energy levels get closer together. Despite its initial success, however, the Bohr model (and successive modifications) has problem in explaining some observations including the spectra of atoms with several electrons. T ...
... of 3.4 eV (first excited level) and so on. As the quantum number n increases the energy levels get closer together. Despite its initial success, however, the Bohr model (and successive modifications) has problem in explaining some observations including the spectra of atoms with several electrons. T ...
Radioactive Decay
... Describe the phenomenon of natural radioactive decay Describe alpha, beta and gamma radiation and their properties In the same way that a rock at a top of hill is not stable and has “too much” potential energy and “wants” to get rid of it by rolling down the hill, the nucleus of an atom can become u ...
... Describe the phenomenon of natural radioactive decay Describe alpha, beta and gamma radiation and their properties In the same way that a rock at a top of hill is not stable and has “too much” potential energy and “wants” to get rid of it by rolling down the hill, the nucleus of an atom can become u ...
ParticleZoo
... ++ produced as short-lived intermediate state, t = 0.5·10-23s corresp. width of state: G = ħ/t = 120 MeV ...
... ++ produced as short-lived intermediate state, t = 0.5·10-23s corresp. width of state: G = ħ/t = 120 MeV ...
Atomic Theory History Chem 11
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
teachers.sd43.bc.ca
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
... positively charged upper plate & a negatively charged bottom plate • A falling, negatively charged oil drop is made to rise between the plates because of exposure to X rays, which ionized some of the drops. There were positive and negative ions • He calculated the charge on the drop by knowing the r ...
UNIT 15: NUCLEUS
... same atomic number Z but different in mass number A. From the definition of isotope, thus the number of protons or electrons are equal but different in the number of neutrons N for two isotopes from the same element. For example : z ...
... same atomic number Z but different in mass number A. From the definition of isotope, thus the number of protons or electrons are equal but different in the number of neutrons N for two isotopes from the same element. For example : z ...
Atomic Structure, the Periodic Table, and Nuclear Radiation
... – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence electron will be less attracted to the nucleus. That’s called shielding. 3. Complet ...
... – The more protons in a nucleus, the more strongly an electron is attracted. 2. Electrons are repelled by other electrons in an atom. So, if other electrons are between a valence electron and the nucleus, the valence electron will be less attracted to the nucleus. That’s called shielding. 3. Complet ...
(a) - decay
... Spontaneous Fission •A large nucleus has a lot of electrostatic repulsion •It would like to separate, but strong forces hold it together •More on this later •It is possible, but rare for it to break apart into two (or more) pieces •Commonly, neutrons are emitted as well. ...
... Spontaneous Fission •A large nucleus has a lot of electrostatic repulsion •It would like to separate, but strong forces hold it together •More on this later •It is possible, but rare for it to break apart into two (or more) pieces •Commonly, neutrons are emitted as well. ...
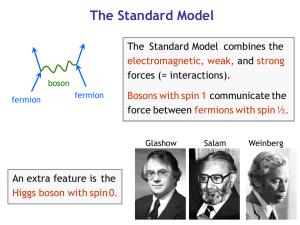
Introduction to Nuclear Forces
... The meson theory of nuclear forces is constructed in analogy with quantum electrodynamics. It is well known that in quantum electrodynamics the electromagnetic field is considered jointly with the particles (photons) associated with it. The field as if consists of photons which are the quanta of thi ...
... The meson theory of nuclear forces is constructed in analogy with quantum electrodynamics. It is well known that in quantum electrodynamics the electromagnetic field is considered jointly with the particles (photons) associated with it. The field as if consists of photons which are the quanta of thi ...