Atomic questions
... Two different models have been developed to explain the existence of atomic energy levels. The Bohr model and the Schrödinger model are both able to predict the principal wavelengths present in the spectrum of atomic hydrogen. ...
... Two different models have been developed to explain the existence of atomic energy levels. The Bohr model and the Schrödinger model are both able to predict the principal wavelengths present in the spectrum of atomic hydrogen. ...
Atomic Theory Lecture
... Since this binds matter together based on mass, we think this plays no role in atoms ...
... Since this binds matter together based on mass, we think this plays no role in atoms ...
Leggi in PDF - SIF Prima Pagina
... to establish the universal nature of these final interactions. The experimental results were discouraging; scattering experiments yielded different final states for each pair of interacting particles. So it happened that these aspects of QCD had to wait until experimentalists themselves came with th ...
... to establish the universal nature of these final interactions. The experimental results were discouraging; scattering experiments yielded different final states for each pair of interacting particles. So it happened that these aspects of QCD had to wait until experimentalists themselves came with th ...
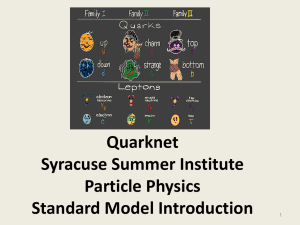
Chapter 4 Presentation File
... shell can hold 2 electrons. 2nd, 3rd, 4th… can hold 8 electrons. ...
... shell can hold 2 electrons. 2nd, 3rd, 4th… can hold 8 electrons. ...
Document
... – each is about 2000 times the mass of the electron, and thus constitutes the vast majority of the mass of a neutral atom (equal number of protons and electrons) – proton has positive charge; mass = 1.007276 a.m.u. – neutron has no charge; mass = 1.008665 a.m.u. – proton by itself (hydrogen nucleus) ...
... – each is about 2000 times the mass of the electron, and thus constitutes the vast majority of the mass of a neutral atom (equal number of protons and electrons) – proton has positive charge; mass = 1.007276 a.m.u. – neutron has no charge; mass = 1.008665 a.m.u. – proton by itself (hydrogen nucleus) ...
Calculating Work and Energy Word Problems
... there for several seconds. How much work is done during the lift? ...
... there for several seconds. How much work is done during the lift? ...
Intro to the Atom PPT
... In the Mid 1800’s is was discovered that when a current of electricity passes through a glass tube a display of colorful lights gradually appears as the gas inside it is evacuated. Research with the tube gave evidence that charged rays of some kind are traversing the tube. . These were called ...
... In the Mid 1800’s is was discovered that when a current of electricity passes through a glass tube a display of colorful lights gradually appears as the gas inside it is evacuated. Research with the tube gave evidence that charged rays of some kind are traversing the tube. . These were called ...
The Electron - webhosting.au.edu
... Nucleus…a dense central core within the atom Proton…positively charged particles in the nucleus Source: whenever “. Came close to a nucleus in the scattering experiment, it experienced a large repulsive and therefore a large deflection. (i.e nucleus is composed of positively charged particles, whic ...
... Nucleus…a dense central core within the atom Proton…positively charged particles in the nucleus Source: whenever “. Came close to a nucleus in the scattering experiment, it experienced a large repulsive and therefore a large deflection. (i.e nucleus is composed of positively charged particles, whic ...
Lab: Atoms and Eggs—Datasheet Name___________________
... Procedure: In this activity an “egg” will represent the nucleus of an atom and different colors of candy will represent the subatomic particles within the nucleus. Write the description of the candy used to represent the subatomic particles based on your teacher’s instructions below: Particle Descri ...
... Procedure: In this activity an “egg” will represent the nucleus of an atom and different colors of candy will represent the subatomic particles within the nucleus. Write the description of the candy used to represent the subatomic particles based on your teacher’s instructions below: Particle Descri ...
Lecture 1.
... The semi-empiric formula describes the bonded energies of the nuclei quiet well. good, usable model ! ...
... The semi-empiric formula describes the bonded energies of the nuclei quiet well. good, usable model ! ...
Periodic Trends - cloudfront.net
... Ions - Ions are charged particles or molecules created through the loss or gain of valence electrons Cation – positively charged particle or molecule created through the loss of valence electrons as a result of ionization Anion – negatively charged particle or molecule created through the gain ...
... Ions - Ions are charged particles or molecules created through the loss or gain of valence electrons Cation – positively charged particle or molecule created through the loss of valence electrons as a result of ionization Anion – negatively charged particle or molecule created through the gain ...
Interactions of Charged Particles with Matter (N Harding)
... The linear energy transfer (LET) is defined as: the amount of energy deposited per unit length (eV/mm) ...
... The linear energy transfer (LET) is defined as: the amount of energy deposited per unit length (eV/mm) ...
Concept Review
... an element are the same, and that atoms from different elements join together to form molecules. 3. The outermost electrons of an atom have greater energy than the innermost electrons of an atom. 4. In both theories, electrons orbit the nucleus and each electron has an energy level associated with i ...
... an element are the same, and that atoms from different elements join together to form molecules. 3. The outermost electrons of an atom have greater energy than the innermost electrons of an atom. 4. In both theories, electrons orbit the nucleus and each electron has an energy level associated with i ...
Chapter 29 - Wayne State University Physics and Astronomy
... Opposite colors attract, red-antired, in analogy with electromagnetism. Different colors also attract though less strongly Residual color force is responsible for nuclear force that bind protrons and neutrons. ...
... Opposite colors attract, red-antired, in analogy with electromagnetism. Different colors also attract though less strongly Residual color force is responsible for nuclear force that bind protrons and neutrons. ...
Nuclear Magnetic Resonance Spectroscopy
... they generate a magnetic field. Nuclei which have an odd number of protons can behave as if they were tiny bar magnets. Of importance to organic chemists is that the hydrogen nucleus, one proton, and the carbon-13 nucleus, 13 protons, both have this property. We will discuss proton magnetic resonanc ...
... they generate a magnetic field. Nuclei which have an odd number of protons can behave as if they were tiny bar magnets. Of importance to organic chemists is that the hydrogen nucleus, one proton, and the carbon-13 nucleus, 13 protons, both have this property. We will discuss proton magnetic resonanc ...
Document
... Going Back to the Akmal, Pandharipande, Ravenhall nuclear phase in in fig 11 of APR ( PRC,58, 1804 (1998)) we find that for the APR [A18 + dv +UIX] the central density of a star of 1.8 solar mass is ( n_B ~0.62 /fm^3), very close to the initial density at which the phase transition begins. The reaso ...
... Going Back to the Akmal, Pandharipande, Ravenhall nuclear phase in in fig 11 of APR ( PRC,58, 1804 (1998)) we find that for the APR [A18 + dv +UIX] the central density of a star of 1.8 solar mass is ( n_B ~0.62 /fm^3), very close to the initial density at which the phase transition begins. The reaso ...