PES Topography - Materials Computation Center
... Minimal Basis Set (MBS) One CBF per occupied orbital on an atom E.g., H has one s function, C has 2s and 1p n-zeta n CBF per occupied orbital on an atom Valence n-zeta MBS for core (1s of C), n-zeta for valence Polarized Add higher angular momentum functions than MBS – e.g., d functions on C Diffuse ...
... Minimal Basis Set (MBS) One CBF per occupied orbital on an atom E.g., H has one s function, C has 2s and 1p n-zeta n CBF per occupied orbital on an atom Valence n-zeta MBS for core (1s of C), n-zeta for valence Polarized Add higher angular momentum functions than MBS – e.g., d functions on C Diffuse ...
Hydrocarbon Compounds
... • contain one or more carbon-carbon double covalent bond – Unsaturated Hydrocarbon • contain less than the maximum number of hydrogen per carbon atom ...
... • contain one or more carbon-carbon double covalent bond – Unsaturated Hydrocarbon • contain less than the maximum number of hydrogen per carbon atom ...
Chapter 11 Theories of Covalent Bonding
... A covalent bond forms when orbitals of two atoms overlap and the overlap region is occupied by two electrons. The greater the overlap the stronger the bond. The stronger the bond the more stable the bond. Orbitals must become oriented so as to obtain the greatest overlap possible. ...
... A covalent bond forms when orbitals of two atoms overlap and the overlap region is occupied by two electrons. The greater the overlap the stronger the bond. The stronger the bond the more stable the bond. Orbitals must become oriented so as to obtain the greatest overlap possible. ...
1 Carbonyl Condensation Reactions (Conjugate Addition) If we look
... In this reaction, an enolate is the nuclophile; one aldehyde molecule becomes the enolate while the other molecule serves as the electrophile. With aldehydes, the equilibrium usually favors the aldol products. But with ketones, the equilibrium usually favors starting materials. Of course, there are ...
... In this reaction, an enolate is the nuclophile; one aldehyde molecule becomes the enolate while the other molecule serves as the electrophile. With aldehydes, the equilibrium usually favors the aldol products. But with ketones, the equilibrium usually favors starting materials. Of course, there are ...
CH 18 blackboard
... By means of hydrogenation reactions, hydrogen is added across the double bonds, converting the unsaturated fat into partially hydrogenated vegetable oil, a saturated fat, which tends to be solid at room temperature. © 2012 Pearson Education, Inc. ...
... By means of hydrogenation reactions, hydrogen is added across the double bonds, converting the unsaturated fat into partially hydrogenated vegetable oil, a saturated fat, which tends to be solid at room temperature. © 2012 Pearson Education, Inc. ...
Chapter 20: Carboxylic Acids and Nitriles
... electrons is the fourth substituent Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
... electrons is the fourth substituent Most amines that have 3 different substituents on N are not resolved because the molecules interconvert by pyramidal inversion ...
Part II - Web site of Dr. Charles Berks
... Another exception occurs between N and O. N has a ½-filled outer 2p subshell from which the ionized electron must be removed whereas for O ionization creates a ½-filled subshell. For O electron-electron repulsion between electrons in the same subshell is being reduced thus ionization of oxygen proc ...
... Another exception occurs between N and O. N has a ½-filled outer 2p subshell from which the ionized electron must be removed whereas for O ionization creates a ½-filled subshell. For O electron-electron repulsion between electrons in the same subshell is being reduced thus ionization of oxygen proc ...
CHEM 208(Organic Chemistry I)
... ORGANIC CHEMISTRY: Laboratory Experiments by Schoffstall, Barbara and Melvin(2nd Edn) Learning Objectives: The principal objective of this course is to get familiar with the C compounds, their nomenclature, functional groups, structures, stereochemistry, their syntheses and reactions. The C compound ...
... ORGANIC CHEMISTRY: Laboratory Experiments by Schoffstall, Barbara and Melvin(2nd Edn) Learning Objectives: The principal objective of this course is to get familiar with the C compounds, their nomenclature, functional groups, structures, stereochemistry, their syntheses and reactions. The C compound ...
Nomenclature of Organic Compounds
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
... b. Two substituents, cite them alphabetically and give the number 1 position to the first substituent. c. Three or more substituents, the substituent given the number 1 is the one that results in a second substituent getting as low a number as possible. ...
Hydrogen Bonding • Aldehydes and ketones don`t hydrogen bond
... The (a) ketone and (b) aldehyde have lost an αhydrogen. In the resulting molecule, the ketone has an alkyl group, while the aldehyde has a hydrogen. Alkyl groups are electron donors, and so it tries to push more electrons to the negative carbanion (red arrow). This makes the molecule less stable tha ...
... The (a) ketone and (b) aldehyde have lost an αhydrogen. In the resulting molecule, the ketone has an alkyl group, while the aldehyde has a hydrogen. Alkyl groups are electron donors, and so it tries to push more electrons to the negative carbanion (red arrow). This makes the molecule less stable tha ...
A STUDY ON STRUCTURAL ASPECTS AND MICROBIAL ACTIVITY OF (E)-4- PYRIDINECARBOXALDEHYDE-3-HYDROXY-5-(HYDROXYMETHYL)-2-METHYL-OXIME
... was done against F2 using SHELXL-97[15]. All non-hydrogen atoms were refined anisotropically and hydrogens were introduced on calculated positions and included in the refinement riding on their respective parent atoms. The pH measurements were made using a digital ELICO electronic model LI 120 pH me ...
... was done against F2 using SHELXL-97[15]. All non-hydrogen atoms were refined anisotropically and hydrogens were introduced on calculated positions and included in the refinement riding on their respective parent atoms. The pH measurements were made using a digital ELICO electronic model LI 120 pH me ...
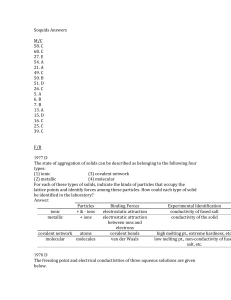
Soquids Answers M/C 58. C 68. C 27. E 54. A 21. A 49. C 50. B 51
... amount of the two solids gives a larger molal solution from the calcium chloride as illustrated by the above equations. (b) Water is more polar than ammonia creating stronger attractions (IMF) between molecules and making it a liquid. (c) Diamond, the hardest naturally occurring substance, has each ...
... amount of the two solids gives a larger molal solution from the calcium chloride as illustrated by the above equations. (b) Water is more polar than ammonia creating stronger attractions (IMF) between molecules and making it a liquid. (c) Diamond, the hardest naturally occurring substance, has each ...
today`s PowerPoint
... • Both aldehydes and ketones will test positively. No other compounds (e.g. Carboxylic acids or esters) will • The precipitate is called 2,4-dinitrophenylhydrazone ...
... • Both aldehydes and ketones will test positively. No other compounds (e.g. Carboxylic acids or esters) will • The precipitate is called 2,4-dinitrophenylhydrazone ...
Παρουσίαση του PowerPoint
... Before and during these syntheses, groups of chemists sitting around blackboards or piles of paper plan the work they are about to undertake. Possible routes are drawn out, criticized, modified again when the behavior of the compounds in the flask turns out to be different from what was expected, un ...
... Before and during these syntheses, groups of chemists sitting around blackboards or piles of paper plan the work they are about to undertake. Possible routes are drawn out, criticized, modified again when the behavior of the compounds in the flask turns out to be different from what was expected, un ...
Chapter 17_CHEM 131
... • The D and L designations do not represent dextrorotatory and levorotatory. • The property of rotating the plane of polarized light is called optical activity, and the molecules with this property are said to be optically active. • Measurements of optical activity are useful for differentiating bet ...
... • The D and L designations do not represent dextrorotatory and levorotatory. • The property of rotating the plane of polarized light is called optical activity, and the molecules with this property are said to be optically active. • Measurements of optical activity are useful for differentiating bet ...
Unit 3 - High School Chemistry
... 2. In general, Ion Sizes Decrease as one move from LEFT to RIGHT of a period WITHIN the METAL GROUPS and WITHIN the NON-METAL GROUPS. This is because the increases in protons in the same row increase the effective nuclear charge (the protons in the nucleus have more pull on the outer electrons, decr ...
... 2. In general, Ion Sizes Decrease as one move from LEFT to RIGHT of a period WITHIN the METAL GROUPS and WITHIN the NON-METAL GROUPS. This is because the increases in protons in the same row increase the effective nuclear charge (the protons in the nucleus have more pull on the outer electrons, decr ...
Molecular Orbital Theory
... • Overlap of hybrid atomic orbitals can form two types of bonds, depending on the geometry of the overlap bonds are formed by “direct” overlap bonds are formed by “parallel” overlay H H H ...
... • Overlap of hybrid atomic orbitals can form two types of bonds, depending on the geometry of the overlap bonds are formed by “direct” overlap bonds are formed by “parallel” overlay H H H ...
1H-NMR and 13C-NMR Spectra - Royal Society of Chemistry
... The 1H-NMR spectrum of 1 shows three multiplets in the aromatic region centered at = 8.30, 9.30 and 9.45 ppm for the 1-H, 2-H and the 2´-H protons (Figure 1), respectively. Downfield shifts were observed for these protons in 3 when compared to 1, which is very similar to that observed in axially a ...
... The 1H-NMR spectrum of 1 shows three multiplets in the aromatic region centered at = 8.30, 9.30 and 9.45 ppm for the 1-H, 2-H and the 2´-H protons (Figure 1), respectively. Downfield shifts were observed for these protons in 3 when compared to 1, which is very similar to that observed in axially a ...
dipole/induced-dipole and dipole/induced
... Halogens are more electronegative than carbon. The C-X bond is therefore polar, with the carbon atom bearing a slight positive and the halogen a slight negative charge. This polarity results in a substantial dipole moment for all the halomethanes and implies that the alkyl halide C-X carbon atom sho ...
... Halogens are more electronegative than carbon. The C-X bond is therefore polar, with the carbon atom bearing a slight positive and the halogen a slight negative charge. This polarity results in a substantial dipole moment for all the halomethanes and implies that the alkyl halide C-X carbon atom sho ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.