Answers, Problem Set 12 (full)... “2,4
... (b) A saturated hydrocarbon is one that contains as many H’s per carbon as possible, and so you could say that it is saturated “with H’s”. It contains no double or triple bonds. Once a double bond or triple bond is formed, there will be less H’s because C only forms 4 bonds before its valence shell ...
... (b) A saturated hydrocarbon is one that contains as many H’s per carbon as possible, and so you could say that it is saturated “with H’s”. It contains no double or triple bonds. Once a double bond or triple bond is formed, there will be less H’s because C only forms 4 bonds before its valence shell ...
كيمياء عضويةc - جامعة دمنهور
... Differentiate the carbonyl group in aldehydes and ketones, and hydroxyl group in alcohols and phenols. Correlate between structure of water with alcohols, phenols, and ethers. Compare the effect of alkyl and aryl groups on the chemistry of characteristics functional groups. Define the electrophilic ...
... Differentiate the carbonyl group in aldehydes and ketones, and hydroxyl group in alcohols and phenols. Correlate between structure of water with alcohols, phenols, and ethers. Compare the effect of alkyl and aryl groups on the chemistry of characteristics functional groups. Define the electrophilic ...
Lab 2 - Academic Computer Center
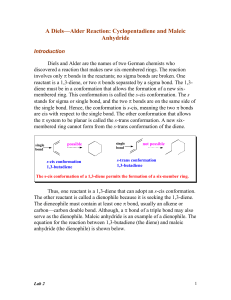
... We saw in previous lessons that some groups are electron donating (EDG) and some are electron withdrawing (EWG). The manner of substitution of electron-donating and electron-withdrawing groups in the reactants governs how easily the reactants produce a Diels-Alder product. When you give someone dir ...
... We saw in previous lessons that some groups are electron donating (EDG) and some are electron withdrawing (EWG). The manner of substitution of electron-donating and electron-withdrawing groups in the reactants governs how easily the reactants produce a Diels-Alder product. When you give someone dir ...
organic compound containing nitrogen
... Chemical properties of Amines : The tendency of nitrogen to share the unpaired electrons underlines the entire chemical behaviour of amines : their basicity, their action as nucleophiles - in both aliphatic and acyl substitution - and the usually high reactivity of aromatic rings bearing amino or su ...
... Chemical properties of Amines : The tendency of nitrogen to share the unpaired electrons underlines the entire chemical behaviour of amines : their basicity, their action as nucleophiles - in both aliphatic and acyl substitution - and the usually high reactivity of aromatic rings bearing amino or su ...
Document
... • They add to each other by opening up their carbon to carbon double bonds. • This process is called addition ...
... • They add to each other by opening up their carbon to carbon double bonds. • This process is called addition ...
Nomenclature of Organic Compounds
... biomolecules such as proteins, lipids, carbohydrates, and nucleic acids. Originally it was believed that these compounds had to come from a living organism, now they are synthesized in the laboratory. The simplest organic compounds are composed of carbon and hydrogen and are known as hydrocarbons. T ...
... biomolecules such as proteins, lipids, carbohydrates, and nucleic acids. Originally it was believed that these compounds had to come from a living organism, now they are synthesized in the laboratory. The simplest organic compounds are composed of carbon and hydrogen and are known as hydrocarbons. T ...
Regents Chemistry
... Know polyatomic ions are groups of atoms covalently bonded that have a positive or negative charge that enables them to form ionic compounds ...
... Know polyatomic ions are groups of atoms covalently bonded that have a positive or negative charge that enables them to form ionic compounds ...
10.3 Alcohols
... longest continuous chain that includes the carbon attached to the -OH group. • Number the carbons in this chain so that the carbon attached to the -OH group has the lowest number. • Drop the -e ending from the name of the parent alkane and replace it with –ol • Alcohols containing two or three -OH g ...
... longest continuous chain that includes the carbon attached to the -OH group. • Number the carbons in this chain so that the carbon attached to the -OH group has the lowest number. • Drop the -e ending from the name of the parent alkane and replace it with –ol • Alcohols containing two or three -OH g ...
I - USC Upstate: Faculty
... d) Bend and stretch couple if stretch bond form 1 side of angle e) Common bond coupling of bending vibrations f) Coupling negligible if groups separated by 1 or more C and vibrations mutually perpendicular C. Fermi Resonance – coupling of fundamental vibrations with overtone or combination 1. 3n-6 f ...
... d) Bend and stretch couple if stretch bond form 1 side of angle e) Common bond coupling of bending vibrations f) Coupling negligible if groups separated by 1 or more C and vibrations mutually perpendicular C. Fermi Resonance – coupling of fundamental vibrations with overtone or combination 1. 3n-6 f ...
2(#pi bonds)
... atoms, we add and subtract the atomic and hybrid orbitals on the adjacent atoms, which are aligned to overlap with each other. • Consider methane, CH4. The sp3 hybrid orbitals of carbon each point to a 1s orbital of hydrogen and, therefore, we add and subtract these atomic orbitals to create molecul ...
... atoms, we add and subtract the atomic and hybrid orbitals on the adjacent atoms, which are aligned to overlap with each other. • Consider methane, CH4. The sp3 hybrid orbitals of carbon each point to a 1s orbital of hydrogen and, therefore, we add and subtract these atomic orbitals to create molecul ...
haloalkanes (halogenoalkanes)
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
Survival Organic Chemistry Part I: Molecular Models
... Use the space below, and on the next page, to draw the Lewis structure for each of the compounds and any structural isomers they may have. ...
... Use the space below, and on the next page, to draw the Lewis structure for each of the compounds and any structural isomers they may have. ...
STEREOCHEMISTRY - M E S KVM College Valanchery.
... 4. Biochemical Processes. Certain bacteria and moulds can destroy one enantiomer of certain racemic modification more rapidly than the other. For example, Pencillium Glaucum, a mould, when grown in a solution of racemic ammonium tartrate, attacks the (+)-form and leaves the (-)-form. This method has ...
... 4. Biochemical Processes. Certain bacteria and moulds can destroy one enantiomer of certain racemic modification more rapidly than the other. For example, Pencillium Glaucum, a mould, when grown in a solution of racemic ammonium tartrate, attacks the (+)-form and leaves the (-)-form. This method has ...
Study Guide for Composition of Matter Test - seys
... - atoms gain one electron (one more electron than protons) = negative charge - larger than the neutral atom = because the extra electron increases the repulsion within the cloud, causing it to expand - chlorine atom (Cl) becomes a negative atom - Chlorine Atom (Cl): 17 electrons (17-) - Chlorine ion ...
... - atoms gain one electron (one more electron than protons) = negative charge - larger than the neutral atom = because the extra electron increases the repulsion within the cloud, causing it to expand - chlorine atom (Cl) becomes a negative atom - Chlorine Atom (Cl): 17 electrons (17-) - Chlorine ion ...
File
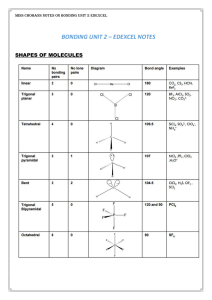
... The shapes of more complicated molecules and ions can also be “explained” by electron pair repulsion theory, or EPR. In applying this principle, we must include both bonding and non-bonding (or “lone”) pairs, and we must count a double or triple bond as if it were one pair (or one region of electron ...
... The shapes of more complicated molecules and ions can also be “explained” by electron pair repulsion theory, or EPR. In applying this principle, we must include both bonding and non-bonding (or “lone”) pairs, and we must count a double or triple bond as if it were one pair (or one region of electron ...
Drawing Electron
... In carbon dioxide, CO2, octets are achieved by sharing two pairs of electrons between atoms; this is called a double bond. ...
... In carbon dioxide, CO2, octets are achieved by sharing two pairs of electrons between atoms; this is called a double bond. ...
Chapter 1: Introduction
... contributes to the diversity and complexity of organic molecules. 15. Distinguish among the three types of isomers: structural, geometric, and enantiomer. 16. Name the major functional groups and describe the chemical properties of the organic molecules in which they occur. 17. List and describe the ...
... contributes to the diversity and complexity of organic molecules. 15. Distinguish among the three types of isomers: structural, geometric, and enantiomer. 16. Name the major functional groups and describe the chemical properties of the organic molecules in which they occur. 17. List and describe the ...
Polarizability
... Dispersion Influence The strength of a dispersion force depends on the ease with which the charge distribution in a molecule can be distorted. ...
... Dispersion Influence The strength of a dispersion force depends on the ease with which the charge distribution in a molecule can be distorted. ...
11-4 Infrared Spectroscopy
... Knowledge of the degree of unsaturation, defined as the numbers of rings and bonds present in a molecule, is useful information when determining the structure of a compound. ...
... Knowledge of the degree of unsaturation, defined as the numbers of rings and bonds present in a molecule, is useful information when determining the structure of a compound. ...
Coordination Chemistry
... each other. It is the electron pair on the slightly negative C that is donated to the metal atom]. The metal eg orbitals are usually empty, but lie along the interuclear axis (d x2-y2 and dz2). These orbitals overlap with the sp-hybrid orbital of a ligand like CO, or the px orbital of ligands like C ...
... each other. It is the electron pair on the slightly negative C that is donated to the metal atom]. The metal eg orbitals are usually empty, but lie along the interuclear axis (d x2-y2 and dz2). These orbitals overlap with the sp-hybrid orbital of a ligand like CO, or the px orbital of ligands like C ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.