CI 12.4 - Sackville School
... The bromine molecule is polarised i.e it has a δ+ and a δ- atom like when the bond is polar. This happens because the electrons in the Br 2 molecule are repelled by the electrons in the C=C. The Brδ+ end now acts as an electrophile. Electrophiles are attracted to a negatively charged region (the C=C ...
... The bromine molecule is polarised i.e it has a δ+ and a δ- atom like when the bond is polar. This happens because the electrons in the Br 2 molecule are repelled by the electrons in the C=C. The Brδ+ end now acts as an electrophile. Electrophiles are attracted to a negatively charged region (the C=C ...
3. Organic Compounds: Alkanes and Cycloalkanes
... Functional group - collection of atoms at a site that have a characteristic behavior in all molecules where it occurs The group reacts in a typical way, generally independent of the rest of the molecule For example, the double bonds in simple and complex alkenes react with bromine in the same way ...
... Functional group - collection of atoms at a site that have a characteristic behavior in all molecules where it occurs The group reacts in a typical way, generally independent of the rest of the molecule For example, the double bonds in simple and complex alkenes react with bromine in the same way ...
3. Organic Compounds: Alkanes and Cycloalkanes
... Functional group - collection of atoms at a site that have a characteristic behavior in all molecules where it occurs The group reacts in a typical way, generally independent of the rest of the molecule For example, the double bonds in simple and complex alkenes react with bromine in the same way ...
... Functional group - collection of atoms at a site that have a characteristic behavior in all molecules where it occurs The group reacts in a typical way, generally independent of the rest of the molecule For example, the double bonds in simple and complex alkenes react with bromine in the same way ...
CH2 Student Revision Guides pdf
... Van der Waals forces are the weak intermolecular forces that exist between all atoms and molecules and include induced-dipole - induced-dipole interactions and dipole-dipole interactions. . The electrons within an atom or molecule are in motion and at a given instant they may be so displaced that th ...
... Van der Waals forces are the weak intermolecular forces that exist between all atoms and molecules and include induced-dipole - induced-dipole interactions and dipole-dipole interactions. . The electrons within an atom or molecule are in motion and at a given instant they may be so displaced that th ...
Document
... the lifeless, primordial Earth. As shown in this recreation, Miller used electrical discharges (simulated lightning) to trigger reactions in a primitive “atmosphere” of H2O, H2, NH3 (ammonia), and CH4 (methane)—some of the gases released by volcanoes ...
... the lifeless, primordial Earth. As shown in this recreation, Miller used electrical discharges (simulated lightning) to trigger reactions in a primitive “atmosphere” of H2O, H2, NH3 (ammonia), and CH4 (methane)—some of the gases released by volcanoes ...
APChapter2-5_ Aug 30-
... disrupts the balance between the two ions. The two ions are very reactive and can drastically ...
... disrupts the balance between the two ions. The two ions are very reactive and can drastically ...
File
... • addition reactions, where atoms are added to each side of the double bond • hydrogenation reactions, where hydrogen atoms are added to each side of the double bond, turning the alkene into an alkane ...
... • addition reactions, where atoms are added to each side of the double bond • hydrogenation reactions, where hydrogen atoms are added to each side of the double bond, turning the alkene into an alkane ...
Chapter 14 – Organic Chemistry
... - study of carbon and carbon compounds Organic Compounds: - compounds which contain both carbon and hydrogen - carbon atoms bond together to form chains, branches, rings, or networks Characteristics of Organic Compounds: - covalent bonding - carbon forms 4 bonds to make a tetrahedron – results in LA ...
... - study of carbon and carbon compounds Organic Compounds: - compounds which contain both carbon and hydrogen - carbon atoms bond together to form chains, branches, rings, or networks Characteristics of Organic Compounds: - covalent bonding - carbon forms 4 bonds to make a tetrahedron – results in LA ...
Chapter 4_Chemical Bonding and Molecular Structure
... A chemical bond formation is attributed to the tendency of a system to attain stability. It was observed that the inertness of noble gases was because of their fully filled outermost orbitals. Hence, it was postulated that the elements having incomplete outermost shells are unstable (reactive). Atom ...
... A chemical bond formation is attributed to the tendency of a system to attain stability. It was observed that the inertness of noble gases was because of their fully filled outermost orbitals. Hence, it was postulated that the elements having incomplete outermost shells are unstable (reactive). Atom ...
SIDE GROUP ADDITION TO THE POLYCYCLIC AROMATIC
... to previously reported alcohol (”OH) and ketone (>C»O) formation. This work represents the first experimental evidence that ice photochemistry may have contributed to the aromatics bearing carbon and nitrogen containing side groups that are detected in primitive meteorites and interplanetary dust par ...
... to previously reported alcohol (”OH) and ketone (>C»O) formation. This work represents the first experimental evidence that ice photochemistry may have contributed to the aromatics bearing carbon and nitrogen containing side groups that are detected in primitive meteorites and interplanetary dust par ...
infra red spectroscopy
... Infra-red spectra are complex due to the many different vibrations taking place in each molecule. Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory. ...
... Infra-red spectra are complex due to the many different vibrations taking place in each molecule. Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory. ...
15iredpp
... Infra-red spectra are complex due to the many different vibrations taking place in each molecule. Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory. ...
... Infra-red spectra are complex due to the many different vibrations taking place in each molecule. Total characterisation of a substance based only on its IR spectrum is almost impossible unless one has computerised data handling facilities for comparison of the obtained spectrum with one in memory. ...
3 center 4 electron bond article
... The three-center, four-electron bond model can also be used as an alternative approach to analysis of the hexacoordinated main group molecules that contain one or two lone pairs of electrons in the central atom, namely, the AB5E or AB4E2 type molecules, where E represents a lone pair. The halogen pe ...
... The three-center, four-electron bond model can also be used as an alternative approach to analysis of the hexacoordinated main group molecules that contain one or two lone pairs of electrons in the central atom, namely, the AB5E or AB4E2 type molecules, where E represents a lone pair. The halogen pe ...
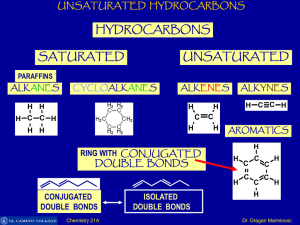
Unsaturated Hydrocarbons
... Benzene boils at 80°C - rather higher than other hydrocarbons of similar molecular size (pentane and hexane, for example). This is presumably due to the ease with which temporary dipoles can be set up involving the delocalized electrons. Methylbenzene (toluene) boils at 111°C. It is a bigger molecul ...
... Benzene boils at 80°C - rather higher than other hydrocarbons of similar molecular size (pentane and hexane, for example). This is presumably due to the ease with which temporary dipoles can be set up involving the delocalized electrons. Methylbenzene (toluene) boils at 111°C. It is a bigger molecul ...
No Slide Title
... Naturally Occurring Oxygen-Containing Compounds • Lipids – Fats and Waxes (also called saponifiable lipids) ...
... Naturally Occurring Oxygen-Containing Compounds • Lipids – Fats and Waxes (also called saponifiable lipids) ...
CH 3
... All enantiomers have a stereogenic center carbon. This makes the molecule chiral having a non-superimposable mirror image. When we name these enantiomers it is necessary to distinguish them from one another. As it turns out each enantiomer in the pair has opposite configuration. Configuration is th ...
... All enantiomers have a stereogenic center carbon. This makes the molecule chiral having a non-superimposable mirror image. When we name these enantiomers it is necessary to distinguish them from one another. As it turns out each enantiomer in the pair has opposite configuration. Configuration is th ...
chapter20(10-27-14)
... • For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. They are isomers of each other - each contains exactly the same number of the same atoms. • The boiling point of the secondary amine is a little lower than the corresponding primary amine wi ...
... • For a fair comparison you would have to compare the boiling point of dimethylamine with that of ethylamine. They are isomers of each other - each contains exactly the same number of the same atoms. • The boiling point of the secondary amine is a little lower than the corresponding primary amine wi ...
protons
... Because of unequal distribution of electrons, water is polar. Negative pole of oxygen is attracted to the positive pole of hydrogen ...
... Because of unequal distribution of electrons, water is polar. Negative pole of oxygen is attracted to the positive pole of hydrogen ...
Aromaticity

In organic chemistry, the term aromaticity is formally used to describe an unusually stable nature of some flat rings of atoms. These structures contain a number of double bonds that interact with each other according to certain rules. As a result of their being so stable, such rings tend to form easily, and once formed, tend to be difficult to break in chemical reactions. Since one of the most commonly encountered aromatic system of compounds in organic chemistry is based on derivatives of the prototypical aromatic compound benzene (common in petroleum), the word “aromatic” is occasionally used to refer informally to benzene derivatives, and this is how it was first defined. Nevertheless, many non-benzene aromatic compounds exist. In living organisms, for example, the most common aromatic rings are the double-ringed bases in RNA and DNA.The earliest use of the term “aromatic” was in an article by August Wilhelm Hofmann in 1855. Hofmann used the term for a class of benzene compounds, many of which do have odors (unlike pure saturated hydrocarbons). Today, there is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds, although in 1855, before the structure of benzene or organic compounds was understood, chemists like Hofmann were beginning to understand that odiferous molecules from plants, such as terpenes, had chemical properties we recognize today are similar to unsaturated petroleum hydrocarbons like benzene.In terms of the electronic nature of the molecule, aromaticity describes the way a conjugated ring of unsaturated bonds, lone pairs of electrons, or empty molecular orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. Aromaticity can be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-bonded to one another. These bonds may be seen as a hybrid of a single bond and a double bond, each bond in the ring identical to every other. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by August Kekulé (see History section below). The model for benzene consists of two resonance forms, which corresponds to the double and single bonds superimposing to produce six one-and-a-half bonds. Benzene is a more stable molecule than would be expected without accounting for charge delocalization.