NUCLEAR CHEMISTRY
... In β− decay, the weak interaction converts a neutron into a proton while emitting an electron *the math must equal on both sides ...
... In β− decay, the weak interaction converts a neutron into a proton while emitting an electron *the math must equal on both sides ...
Lithium 6.941 - mrkearsley.com
... Fusion is a nuclear reaction where two light atomic nuclei fuse or combine to form a single heavier nucleus. ...
... Fusion is a nuclear reaction where two light atomic nuclei fuse or combine to form a single heavier nucleus. ...
Nuclear Notes
... small quantity of energy (so a small amount of mass is lost and some energy is released). The mass lost is called the mass defect. Nuclear energies are generally expressed in electron-volts. One electron-volt (eV) is the energy an electron acquires as it moves through a potential difference of 1 vol ...
... small quantity of energy (so a small amount of mass is lost and some energy is released). The mass lost is called the mass defect. Nuclear energies are generally expressed in electron-volts. One electron-volt (eV) is the energy an electron acquires as it moves through a potential difference of 1 vol ...
Unit #12: Nuclear Chemistry
... •The left side (reactants side) of equation and the right side (products side) of equation ...
... •The left side (reactants side) of equation and the right side (products side) of equation ...
Nuclear Chemistry
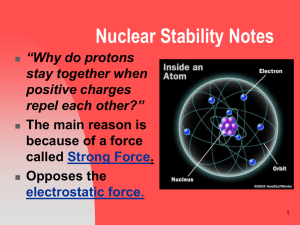
... We know that like charges repel each other, yet a nucleus can have dozens of positively charged protons held together. Why? Neutrons are a major reason. All nuclei with 2 or more protons have neutrons. The neutrons and the protons meld by a force of nature, different than gravity or electromagne ...
... We know that like charges repel each other, yet a nucleus can have dozens of positively charged protons held together. Why? Neutrons are a major reason. All nuclei with 2 or more protons have neutrons. The neutrons and the protons meld by a force of nature, different than gravity or electromagne ...
Radioactivity and Nuclear Reactions
... • The strong force is about 100 x greater than the electric force (the force that would cause the protons to repel each other). • The strong force is a short range force that quickly becomes extremely weak as protons and neutrons get farther apart. ...
... • The strong force is about 100 x greater than the electric force (the force that would cause the protons to repel each other). • The strong force is a short range force that quickly becomes extremely weak as protons and neutrons get farther apart. ...
Lab Science 9 Pacing Guide
... 6. Explain that the electric force between the nucleus and the electrons hold an atom together. Relate that on a larger scale, electric forces hold solid and liquid materials together (e.g., salt crystals and water). 7. Show how atoms may be bonded together by losing, gaining or sharing electrons an ...
... 6. Explain that the electric force between the nucleus and the electrons hold an atom together. Relate that on a larger scale, electric forces hold solid and liquid materials together (e.g., salt crystals and water). 7. Show how atoms may be bonded together by losing, gaining or sharing electrons an ...
Nuclear chemistry – the study of nuclear reactions and their uses in
... ii. Greater amounts of energy are released if very light nuclei are combined or fused together to give more massive nuclei 1. This process is call fusion (exothermic process) and is the essential energy producing process in the Sun. ...
... ii. Greater amounts of energy are released if very light nuclei are combined or fused together to give more massive nuclei 1. This process is call fusion (exothermic process) and is the essential energy producing process in the Sun. ...
Topic 7 Atomic and Nuclear Physics
... Nucleon number (A) The number of protons plus neutrons in a nucleus Proton number (Z): The number of protons in a nucleus. Neutron number (N): The number of neutrons in a nucleus. Radioactive half-life: The time taken for half the original nuclei in a sample to decay. Artificial (induced) transmutat ...
... Nucleon number (A) The number of protons plus neutrons in a nucleus Proton number (Z): The number of protons in a nucleus. Neutron number (N): The number of neutrons in a nucleus. Radioactive half-life: The time taken for half the original nuclei in a sample to decay. Artificial (induced) transmutat ...
Nuclear Stability Notes
... and works within a very short distance. Neutrons act as insulation, since they have no charge, but have the strong force to bring other nucleons (protons and neutrons) together. ...
... and works within a very short distance. Neutrons act as insulation, since they have no charge, but have the strong force to bring other nucleons (protons and neutrons) together. ...
File
... Nuclear Fusion: The combining of atomic nuclei to form a larger atom is called fusion Nuclear fusion occurs in the sun where hydrogen atoms 1 helium fuse to form ...
... Nuclear Fusion: The combining of atomic nuclei to form a larger atom is called fusion Nuclear fusion occurs in the sun where hydrogen atoms 1 helium fuse to form ...
Foldable - Georgetown ISD
... Gamma (γ) Rays (*deadliest form of radiation) – no particle (only radiation/energy) Shielding: thick layers of lead or concrete Fission: *splitting an atom into smaller atoms (also releases energy), *a very heavy nucleus splits into more stable, intermediate-size nuclei Uses: nuclear power plants (* ...
... Gamma (γ) Rays (*deadliest form of radiation) – no particle (only radiation/energy) Shielding: thick layers of lead or concrete Fission: *splitting an atom into smaller atoms (also releases energy), *a very heavy nucleus splits into more stable, intermediate-size nuclei Uses: nuclear power plants (* ...
Syllabus overview
... radiation on structures within cells. A simple account of short‑term and long‑term effects of radiation on the body is required. 7.2.5 Explain why some nuclei are stable while others are unstable. An explanation in terms of relative numbers of protons and neutrons and the forces involved is all that ...
... radiation on structures within cells. A simple account of short‑term and long‑term effects of radiation on the body is required. 7.2.5 Explain why some nuclei are stable while others are unstable. An explanation in terms of relative numbers of protons and neutrons and the forces involved is all that ...
Document
... Like this • Starting nuclear chain reactions = releases a huge amount of energy (E) • Per unit volume, an atom bomb may be millions or billions of times more powerful than TNT. • Nuclear reactions (rxn) occur: neutrons r fired @ closely packed atoms w/ heavy nuclei (uranium or plutonium isotopes). ...
... Like this • Starting nuclear chain reactions = releases a huge amount of energy (E) • Per unit volume, an atom bomb may be millions or billions of times more powerful than TNT. • Nuclear reactions (rxn) occur: neutrons r fired @ closely packed atoms w/ heavy nuclei (uranium or plutonium isotopes). ...
Chapter 19 Nuclear Chemistry
... The Kinetics of Radioactive Decay Nuclear Transformations Detection and Uses of Radioactivity Thermodynamic Stability of the Nucleus Nuclear Fission and Nuclear Fusion Effects of Radiation ...
... The Kinetics of Radioactive Decay Nuclear Transformations Detection and Uses of Radioactivity Thermodynamic Stability of the Nucleus Nuclear Fission and Nuclear Fusion Effects of Radiation ...
Chapter 1 Learning Objective Summary
... 6. Learn to balance common nuclear reactions, know the common radioactive particles involved, and understand fission and fusion Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that ...
... 6. Learn to balance common nuclear reactions, know the common radioactive particles involved, and understand fission and fusion Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that ...
Nuclear Chemistry
... Radioactive • All nuclei with atomic numbers greater than 83 are radioactive • These nuclei have both too many neutrons and too many protons to be stable – So most undergo decay ...
... Radioactive • All nuclei with atomic numbers greater than 83 are radioactive • These nuclei have both too many neutrons and too many protons to be stable – So most undergo decay ...
Nuclear Astrophysics (1)
... the observing time. Then the following relation holds between the chemical potentials. ...
... the observing time. Then the following relation holds between the chemical potentials. ...
Advanced Version
... d. Plan and carry out investigations on the effects of heat transfer on molecular motion as it relates to the collision of atoms (conduction), through space (radiation), or in currents in a liquid or a gas (convection). ...
... d. Plan and carry out investigations on the effects of heat transfer on molecular motion as it relates to the collision of atoms (conduction), through space (radiation), or in currents in a liquid or a gas (convection). ...
Terms to Know
... Positrons : The positron is the antiparticle of the electron. It has the same mass and the same quantity of electric charge as does the electron, but its electric charge is positive rather than negative. Radioactivity : Radioactivity is the emission of radiation by unstable nuclei. That radiation ma ...
... Positrons : The positron is the antiparticle of the electron. It has the same mass and the same quantity of electric charge as does the electron, but its electric charge is positive rather than negative. Radioactivity : Radioactivity is the emission of radiation by unstable nuclei. That radiation ma ...
Click here for printer-friendly sample test questions
... A. are responsible for the formation of most elements. B. are commonly used in nuclear power plants. C. are used in Russian-style nuclear reactors. D. occur when electrons combine with neutrons. 3. Nuclear fission reactions A. are responsible for the formation of most elements. B. are commonly used ...
... A. are responsible for the formation of most elements. B. are commonly used in nuclear power plants. C. are used in Russian-style nuclear reactors. D. occur when electrons combine with neutrons. 3. Nuclear fission reactions A. are responsible for the formation of most elements. B. are commonly used ...
Ch 21.1 Study Guide
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
... 1. _____ Based on the information about the three elementary particles on page 683 of the text, which has the greatest mass? (a) the proton (b) the neutron (c) the electron (d) They all have the same mass. 2. _____ The force that keeps nucleons together is (a) a strong nuclear force. (b) a weak nucl ...
Elements!
... • Atomic Model has changed dramatically through history •Subatomic particles make up all atoms ...
... • Atomic Model has changed dramatically through history •Subatomic particles make up all atoms ...
Nuclear fusion

In nuclear physics, nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new nucleus. During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy). Fusion is the process that powers active or ""main sequence"" stars.The fusion of two nuclei with lower masses than Iron-56 (which, along with Nickel-62, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements. There are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernova.Following the discovery of quantum tunneling by Friedrich Hund, in 1929 Robert Atkinson and Fritz Houtermans used the measured masses of light elements to predict that large amounts of energy could be released by fusing small nuclei. Building upon the nuclear transmutation experiments by Ernest Rutherford, carried out several years earlier, the laboratory fusion of hydrogen isotopes was first accomplished by Mark Oliphant in 1932. During the remainder of that decade the steps of the main cycle of nuclear fusion in stars were worked out by Hans Bethe. Research into fusion for military purposes began in the early 1940s as part of the Manhattan Project. Fusion was accomplished in 1951 with the Greenhouse Item nuclear test. Nuclear fusion on a large scale in an explosion was first carried out on November 1, 1952, in the Ivy Mike hydrogen bomb test.Research into developing controlled thermonuclear fusion for civil purposes also began in earnest in the 1950s, and it continues to this day. The present article is about the theory of fusion. For details of the quest for controlled fusion and its history, see the article Fusion power.