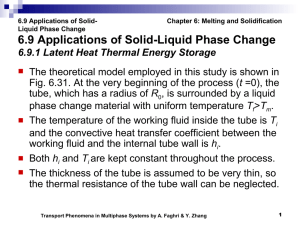
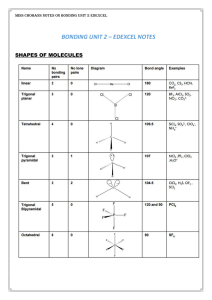
Introduction to Alloy Phase Diagrams
... is a hypereutectic alloy (meaning "greater than"). In the eutectic system described above, the two components of the system have the same crystal structure. This, and other factors, allows complete miscibilitybetween them. Eutectic systems, however, also can be formed by two components having differ ...
... is a hypereutectic alloy (meaning "greater than"). In the eutectic system described above, the two components of the system have the same crystal structure. This, and other factors, allows complete miscibilitybetween them. Eutectic systems, however, also can be formed by two components having differ ...
DUE: Tuesday, Jan. 20, 2015 Solutions Take Home Test
... c. the oxygen atom d. There is no difference in the electronegativities of the atoms in a water molecule. ...
... c. the oxygen atom d. There is no difference in the electronegativities of the atoms in a water molecule. ...
Relative Atomic Masses
... these three compounds. But this does not seem likely, since it is hard to understand the fixed mass proportions without thinking that we are combining particles with fixed mass proportions. Still, we must have made an incorrect assumption since our conclusions were contradictory. Recall that in doin ...
... these three compounds. But this does not seem likely, since it is hard to understand the fixed mass proportions without thinking that we are combining particles with fixed mass proportions. Still, we must have made an incorrect assumption since our conclusions were contradictory. Recall that in doin ...
1 SOLUTIONS
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
... · The Solubility of gases decreases with increasing temperature (the colder the water, the better the gases dissolve). Examples are: Ø soda water keeps its carbonation (CO2 gas) better at low temperatures (at room temperature they go “flat” faster) Ø cold oceans have a higher concentration ...
Electron spin echo studies
... plane and bridging a carbon-carbon bond on the graphene surface; 共c兲 a carbon vacancy formed by removing a carbon atom from graphene sheet and relaxing to the pentagon structure;17 共d兲 sterically protected carbon radicals which are immobilized in the aromatic system of sp2 bonded carbons;18 and 共e兲 ...
... plane and bridging a carbon-carbon bond on the graphene surface; 共c兲 a carbon vacancy formed by removing a carbon atom from graphene sheet and relaxing to the pentagon structure;17 共d兲 sterically protected carbon radicals which are immobilized in the aromatic system of sp2 bonded carbons;18 and 共e兲 ...
Inequalities for Schrödinger Operators and
... Heisenberg’s uncertainty principle is not very useful in practice, however. Specifically, while a small value of (ψ, x2 ψ) means that ψ is localized close to the origin, a large value (ψ, x2 ψ) does not mean it is spread out. In fact, (ψ, x2 ψ) could be huge even if most of the mass of ψ is localize ...
... Heisenberg’s uncertainty principle is not very useful in practice, however. Specifically, while a small value of (ψ, x2 ψ) means that ψ is localized close to the origin, a large value (ψ, x2 ψ) does not mean it is spread out. In fact, (ψ, x2 ψ) could be huge even if most of the mass of ψ is localize ...
Word - IUPAC Task Group on Atmospheric Chemical Kinetic Data
... Therefore, for practical purposes (e.g., using the constants to calculate coverages) reporting consistently in the form of KlinC has the advantage that no inherent assumption about Nmax has to be made to derive partitioning from the experiments at low pressures. The tabulated values of partition coe ...
... Therefore, for practical purposes (e.g., using the constants to calculate coverages) reporting consistently in the form of KlinC has the advantage that no inherent assumption about Nmax has to be made to derive partitioning from the experiments at low pressures. The tabulated values of partition coe ...
Phys. Rev
... What is a quantum phase transition ? Non-analyticity in ground state properties as a function of some control parameter g ...
... What is a quantum phase transition ? Non-analyticity in ground state properties as a function of some control parameter g ...
Part V The Third Law and Free Energy
... a disordered pattern as CO CO CO OC CO, then this crystal may have this disorder 'frozen in' as the temperature is lowered because there will be very little thermal energy available for the molecules to rearrange to the ordered state. In that case this randomness will provide two states to each mole ...
... a disordered pattern as CO CO CO OC CO, then this crystal may have this disorder 'frozen in' as the temperature is lowered because there will be very little thermal energy available for the molecules to rearrange to the ordered state. In that case this randomness will provide two states to each mole ...
Physical Science - Garfield School District
... Pressure is the amount of force exerted on a given area of surface. All fluids exert an upward buoyant force on matter. Pascal’s principle states that a change in pressure at any point in an enclosed fluid will be transmitted equally to all parts of the fluid. Fluids move faster through small areas ...
... Pressure is the amount of force exerted on a given area of surface. All fluids exert an upward buoyant force on matter. Pascal’s principle states that a change in pressure at any point in an enclosed fluid will be transmitted equally to all parts of the fluid. Fluids move faster through small areas ...
SOLID STATE CHEMISTRY Lecture/Lession Plan
... i) Conductors: The solid materials through which electricity can pass easily are called conductors. Metals are very good conductor of electricity. Silver metal is the best conductor among the metals. The free electrons present in the metal are responsible for the conduction. For this reason metals a ...
... i) Conductors: The solid materials through which electricity can pass easily are called conductors. Metals are very good conductor of electricity. Silver metal is the best conductor among the metals. The free electrons present in the metal are responsible for the conduction. For this reason metals a ...
nainan k. varghese
... the all-encompassing universal medium that pervades entire space, outside 3D matter-particles. Author explains a wide array of physical phenomena from origin of 3D matter to gravity and subatomic interactions to cosmological events, using but simple mechanical interactions of quanta of matter. There ...
... the all-encompassing universal medium that pervades entire space, outside 3D matter-particles. Author explains a wide array of physical phenomena from origin of 3D matter to gravity and subatomic interactions to cosmological events, using but simple mechanical interactions of quanta of matter. There ...
Disorder in two-dimensional Josephson junctions
... Department of Physics, Ben-Gurion University, Beer-Sheva, 84105, Israel ~Received 18 July 1996! An effective free energy of a two-dimensional ~i.e., large area! Josephson junction is derived, allowing for thermal fluctuations, random magnetic fields, and external currents. We show by using replica-s ...
... Department of Physics, Ben-Gurion University, Beer-Sheva, 84105, Israel ~Received 18 July 1996! An effective free energy of a two-dimensional ~i.e., large area! Josephson junction is derived, allowing for thermal fluctuations, random magnetic fields, and external currents. We show by using replica-s ...
Group–A Thermodynamics - New Age International
... can flow) and if there is no spontaneous change in any of the properties of the system, the system is said to exist in thermal equilibrium with its surroundings. Also, a system may exist in thermal equilibrium with another system. Adjacent figure 1.4 illustrates thermal equilibrium between two syste ...
... can flow) and if there is no spontaneous change in any of the properties of the system, the system is said to exist in thermal equilibrium with its surroundings. Also, a system may exist in thermal equilibrium with another system. Adjacent figure 1.4 illustrates thermal equilibrium between two syste ...
Coupled Microbial and Transport Processes in Soils
... directions of anisotropy are aligned with the coordinate system so that the cross components of the k tensor are zero. If coupled, multifluid flow is considered, equations similar to Eq. [1] can also be written to describe the mass conservation of air and nonaqueous phase liquids (White and Oostrom, ...
... directions of anisotropy are aligned with the coordinate system so that the cross components of the k tensor are zero. If coupled, multifluid flow is considered, equations similar to Eq. [1] can also be written to describe the mass conservation of air and nonaqueous phase liquids (White and Oostrom, ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).