CHEM1100 Practice Exam 2 You have 120 minutes to complete this
... 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl–, Br–, I– are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. 4. Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually a ...
... 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl–, Br–, I– are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. 4. Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually a ...
The Atomic Molecular Theory
... These data help justify an atomic view of matter. We can simply argue that, for example, lead sul de is formed by taking one lead atom and combining it with one sulfur atom. If this were true, then we also must conclude that the ratio of the mass of a lead atom to that of a sulfur atom is the same a ...
... These data help justify an atomic view of matter. We can simply argue that, for example, lead sul de is formed by taking one lead atom and combining it with one sulfur atom. If this were true, then we also must conclude that the ratio of the mass of a lead atom to that of a sulfur atom is the same a ...
Acid Base Balance - faculty at Chemeketa
... ion is released (OH-) this little guy actively seeks out and attaches itself to acids floating around in the blood-stream. Bicarbonate ...
... ion is released (OH-) this little guy actively seeks out and attaches itself to acids floating around in the blood-stream. Bicarbonate ...
Model Description Sheet
... synthesis and treat prostate, breast, and other hormone responsive cancers. Cholesterol is the precursor of all steroid hormones including testosterone and estrogen. CYP17A1, an enzyme bound to the membrane of adrenal cells, plays a critical role in the biosynthesis of steroid hormones. This enzyme ...
... synthesis and treat prostate, breast, and other hormone responsive cancers. Cholesterol is the precursor of all steroid hormones including testosterone and estrogen. CYP17A1, an enzyme bound to the membrane of adrenal cells, plays a critical role in the biosynthesis of steroid hormones. This enzyme ...
Summer Assignment
... 5. All hydroxides are insoluble except compounds of the alkali metals, Ca2+, Sr2+,and Ba2+. 6. All compounds containing PO43-, S2-, CO32-, and SO32- ions are insoluble except those that also contain alkali metals or NH4+. ...
... 5. All hydroxides are insoluble except compounds of the alkali metals, Ca2+, Sr2+,and Ba2+. 6. All compounds containing PO43-, S2-, CO32-, and SO32- ions are insoluble except those that also contain alkali metals or NH4+. ...
IB BIOLOGY: Respiration Notes - NatronaBiology-IB2
... matrix and inner membrane/cristae of the mitochondrion. The pumps are reduced, giving them energy to pump protons into the inner membrane space. The electrons are transferred along a chain of pumps, continuously losing energy. The proton pumps create a high concentration gradient of protons (H+) ins ...
... matrix and inner membrane/cristae of the mitochondrion. The pumps are reduced, giving them energy to pump protons into the inner membrane space. The electrons are transferred along a chain of pumps, continuously losing energy. The proton pumps create a high concentration gradient of protons (H+) ins ...
Respiratory Physiology
... As arterial blood flows through capillaries, 5 ml oxygen are released. The saturation of hemoglobin in arterial blood explains why breathing deeply has little effect on oxygen saturation in hemoglobin Hemoglobin is almost completely saturated at a PO2 of 70 mm Hg, further increases in PO2 produce on ...
... As arterial blood flows through capillaries, 5 ml oxygen are released. The saturation of hemoglobin in arterial blood explains why breathing deeply has little effect on oxygen saturation in hemoglobin Hemoglobin is almost completely saturated at a PO2 of 70 mm Hg, further increases in PO2 produce on ...
SUCCINYL-CoA SYNTHETASE from a prokaryote (Lot 140901b)
... The enzyme is supplied as an ammonium sulphate suspension and should be stored at 4°C. For assay, this enzyme should be diluted in 100 mM glycylglycine buffer, pH 8.4 containing 10 mM MgCl2. Swirl to mix the enzyme suspension immediately prior to use. ...
... The enzyme is supplied as an ammonium sulphate suspension and should be stored at 4°C. For assay, this enzyme should be diluted in 100 mM glycylglycine buffer, pH 8.4 containing 10 mM MgCl2. Swirl to mix the enzyme suspension immediately prior to use. ...
Free Radicals and other reactive species in Disease
... Low levels of oxidative base damage products are present in DNA isolated from all aerobic cells; levels often increase in patients with chronic inflammatory diseases or subjected to oxidative stress, e.g. from smoking. Some base damage products are excreted in urine, presumably resulting from DNA rep ...
... Low levels of oxidative base damage products are present in DNA isolated from all aerobic cells; levels often increase in patients with chronic inflammatory diseases or subjected to oxidative stress, e.g. from smoking. Some base damage products are excreted in urine, presumably resulting from DNA rep ...
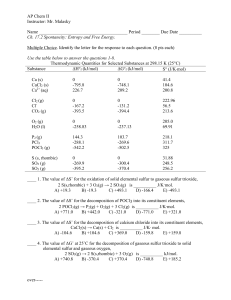
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
X012/11/02
... provided in this answer book, and must be written clearly and legibly in ink. Rough work, if any should be necessary, should be written in this book, and then scored through when the fair copy has been written. If further space is required, a supplementary sheet for rough work may be obtained from t ...
... provided in this answer book, and must be written clearly and legibly in ink. Rough work, if any should be necessary, should be written in this book, and then scored through when the fair copy has been written. If further space is required, a supplementary sheet for rough work may be obtained from t ...
The s-Block Elements - GCG-42
... Reactions of hydrides They all react readily with water to give the metal hydroxide and hydrogen due to the strong basic property of the hydride ion, H:H:-(s)+ H2O(l) H2(g)+ OH-(aq) Hydride ions are also good reducing agent. They can be used to prepare complex hydrides such as LiAlH4 and NaBH4 wh ...
... Reactions of hydrides They all react readily with water to give the metal hydroxide and hydrogen due to the strong basic property of the hydride ion, H:H:-(s)+ H2O(l) H2(g)+ OH-(aq) Hydride ions are also good reducing agent. They can be used to prepare complex hydrides such as LiAlH4 and NaBH4 wh ...
Myocardial infarction - Lectures For UG-5
... therefore hemolysis must be avoided LDH has its poorest stability at 0°C Clinical Significance In myocardial infarction, LD increases 3-12 hours after the onset of pain Peaks at 48-60 hours and remain elevated for 10-14 days In MI, LD1 is higher than LD2, thus called “flipped” LD ...
... therefore hemolysis must be avoided LDH has its poorest stability at 0°C Clinical Significance In myocardial infarction, LD increases 3-12 hours after the onset of pain Peaks at 48-60 hours and remain elevated for 10-14 days In MI, LD1 is higher than LD2, thus called “flipped” LD ...
Cellular respiration *vs
... This fermentation occurs in the cytoplasm of the cell and produces only 2 molecules of ATP—Therefore, this is not as efficient of an energy path to follow. • So where does this fermentation occur—have you every been sore after a hard workout or from playing a sport you have not done for a long time? ...
... This fermentation occurs in the cytoplasm of the cell and produces only 2 molecules of ATP—Therefore, this is not as efficient of an energy path to follow. • So where does this fermentation occur—have you every been sore after a hard workout or from playing a sport you have not done for a long time? ...
READ MORE - MindBody Medicine Center
... molecule? First of all, the vitamins, minerals, complex carbohydrates, proteins and fats come from our diet and provide the building blocks to citric acid cycle energy production. If any one of the n ...
... molecule? First of all, the vitamins, minerals, complex carbohydrates, proteins and fats come from our diet and provide the building blocks to citric acid cycle energy production. If any one of the n ...
Chap. 4 - Chemical Reactions
... periodic table, fluorine being the most reactive. Consider the following example: ...
... periodic table, fluorine being the most reactive. Consider the following example: ...
Student Background Information 1C
... Observing carefully, attempt to arrange the lung layers in the correct order. Each part of the respiratory system is designed for the specific job it does and you can often guess what the job is by observing – you will be surprised at what you can do when you take time to observe and think about wha ...
... Observing carefully, attempt to arrange the lung layers in the correct order. Each part of the respiratory system is designed for the specific job it does and you can often guess what the job is by observing – you will be surprised at what you can do when you take time to observe and think about wha ...
How Air Moves In and Out of the Lung
... . Which of the statements below gives the best definition of gas exchange? :d) exchanging oxygen for carbon dioxide in the lung alveoli. 10. Which of the following help the lungs to be such good gas exchange organs? (Choose at least 4). They have a large surface area. The air in the alveoli and bloo ...
... . Which of the statements below gives the best definition of gas exchange? :d) exchanging oxygen for carbon dioxide in the lung alveoli. 10. Which of the following help the lungs to be such good gas exchange organs? (Choose at least 4). They have a large surface area. The air in the alveoli and bloo ...
Gas Exchange Activity - Delaware Access Project
... Creating models is one way to understand and communicate scientific information. ...
... Creating models is one way to understand and communicate scientific information. ...
7-Keto DHEA - Scientific Bio
... two hormones. Since DHEA and 7-Keto DHEA levels decrease faster with age than cortisol, this creates an imbalance in the body and may cause a decline in cellular immunity. Supplementing with 7-Keto DHEA can improve immune system functioning by counteracting the negative effects that chronic high lev ...
... two hormones. Since DHEA and 7-Keto DHEA levels decrease faster with age than cortisol, this creates an imbalance in the body and may cause a decline in cellular immunity. Supplementing with 7-Keto DHEA can improve immune system functioning by counteracting the negative effects that chronic high lev ...
Unit 6 Chemical Equations and Reactions Balancing Equations
... 4. Sodium metal is added to sulfuric acid and produce sodium sulfate and hydrogen. 2 Na (s) + H2SO4 (aq) → Na2SO4 + H2 (g) Single Replacement 5. White phosphorus (P4) reacts with chlorine to make phosphorus trichloride P4 (s) + 6 Cl2 (g) → 4 PCl3 (g) Synthesis 6. Magnesium chlorate, is heated strong ...
... 4. Sodium metal is added to sulfuric acid and produce sodium sulfate and hydrogen. 2 Na (s) + H2SO4 (aq) → Na2SO4 + H2 (g) Single Replacement 5. White phosphorus (P4) reacts with chlorine to make phosphorus trichloride P4 (s) + 6 Cl2 (g) → 4 PCl3 (g) Synthesis 6. Magnesium chlorate, is heated strong ...
AP Chemistry Summer Assignment
... 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gases are measured at the same tempe ...
... 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of sulfur dioxide would be produced from 10.0 l of Oxygen? Assume 100% yield and that all gases are measured at the same tempe ...
File
... • photosynthesis & cellular respiration are cyclic processes • the products of one are the reactants of the other ...
... • photosynthesis & cellular respiration are cyclic processes • the products of one are the reactants of the other ...