Unit 8 Packet - Page 1 of 18 Honors Chemistry
... When writing equations you must satisfy the law of conservation matter (matter can not be created or destroyed) Therefore we must have the same type and number of each atom on each side of the equation. BALANCING EQUATIONS: 4 steps: 1. Start with a word equation 2. Convert to a formula equation (don ...
... When writing equations you must satisfy the law of conservation matter (matter can not be created or destroyed) Therefore we must have the same type and number of each atom on each side of the equation. BALANCING EQUATIONS: 4 steps: 1. Start with a word equation 2. Convert to a formula equation (don ...
MS Word Version
... acid R-groups packed into the interior of the molecule are predominantly hydrophobic in character while those exposed on the surface of the molecule are generally hydrophilic, thus making the molecule relatively water soluble. Each myoglobin molecule contains one heme prosthetic group inserted into ...
... acid R-groups packed into the interior of the molecule are predominantly hydrophobic in character while those exposed on the surface of the molecule are generally hydrophilic, thus making the molecule relatively water soluble. Each myoglobin molecule contains one heme prosthetic group inserted into ...
I. Introduction
... __________________________________________________________________ 11. Surface tension is ________________________________________________ 12. Surfactant is located ___________________________________________ and functions to ________________________________________________________ 13. If a person n ...
... __________________________________________________________________ 11. Surface tension is ________________________________________________ 12. Surfactant is located ___________________________________________ and functions to ________________________________________________________ 13. If a person n ...
chapter 1: the lungs and respiratory system
... systems of the body. Changes in the body trigger complex responses. Various body systems often work together to return the internal environment to a normal state. ...
... systems of the body. Changes in the body trigger complex responses. Various body systems often work together to return the internal environment to a normal state. ...
Hemoglobin and O2 transport - SHMD 339: Exercise Physiology 3
... - myoglobin- O2 stores must be replenished to ensure O2 is available for the next time exercise ...
... - myoglobin- O2 stores must be replenished to ensure O2 is available for the next time exercise ...
File - Ms. Richards IB Biology HL
... d) Note that a pO2 of 40 mm Hg, the average pO2 of tissue cells at rest, only 25% of the available oxygen splits from Hb and is used. (Big reserve of oxygen) e) Several other factors influence the affinity of Hb for oxygen, the strength of the Hb-O2 binding. Keep in mind that metabolically active ce ...
... d) Note that a pO2 of 40 mm Hg, the average pO2 of tissue cells at rest, only 25% of the available oxygen splits from Hb and is used. (Big reserve of oxygen) e) Several other factors influence the affinity of Hb for oxygen, the strength of the Hb-O2 binding. Keep in mind that metabolically active ce ...
Chapter 20 – The Representative Elements
... reactions of nitric acid with metals do not produce H2 gas. This is because the nitrate ion, NO3-, is a stronger oxidizing agent; therefore, redox reactions involving the nitrate ions occurs before one that involve the H+ ions. The products of the reactions with metals vary with the metal reactivity ...
... reactions of nitric acid with metals do not produce H2 gas. This is because the nitrate ion, NO3-, is a stronger oxidizing agent; therefore, redox reactions involving the nitrate ions occurs before one that involve the H+ ions. The products of the reactions with metals vary with the metal reactivity ...
AP Chemistry Summer Assignment 2016 revised
... h.Nitrogen gas reacts with Hydrogen to form Ammonia. i.Hydrogen reacts with Iodine gas to form Hydrogen Iodide. j. Sodium reacts with Iodine gas to form Sodium Iodide. k.Carbon dioxide combines with water to form carbonic acid. l.Magnesium and nitrogen gas combine to form magnesium nitride. m.Conc. ...
... h.Nitrogen gas reacts with Hydrogen to form Ammonia. i.Hydrogen reacts with Iodine gas to form Hydrogen Iodide. j. Sodium reacts with Iodine gas to form Sodium Iodide. k.Carbon dioxide combines with water to form carbonic acid. l.Magnesium and nitrogen gas combine to form magnesium nitride. m.Conc. ...
Lecture No. 7
... • HCN is very poisonous, but in plants occurs in quite low concentrations • Additionally, releasing process is slow so there is not sufficient amount cumulated in one time • Lower concentration of amygdalin is found in plums, blackthorns, cherries, sour cherries and apples. ...
... • HCN is very poisonous, but in plants occurs in quite low concentrations • Additionally, releasing process is slow so there is not sufficient amount cumulated in one time • Lower concentration of amygdalin is found in plums, blackthorns, cherries, sour cherries and apples. ...
4.IonicCompounds - Gleneaglesunit1and2chemistry2012
... Types of Chemical Bonds • Ionic bonds formed when metal atoms combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
... Types of Chemical Bonds • Ionic bonds formed when metal atoms combined with non-metal atoms • Metallic bonds formed when metal atoms combined with metal atoms. • Covalent bonds formed when non-metal atoms combined with non-metal atoms. ...
Respiration - Orange Coast College
... • The slides in this presentation were originally created by Marc C. Perkins (http://faculty.orangecoastcollege.edu/mperkins). • You are free to use, modify, and distribute these slides according to the terms of the Creative Commons license (e.g., you must attribute the slides, no commercial uses ar ...
... • The slides in this presentation were originally created by Marc C. Perkins (http://faculty.orangecoastcollege.edu/mperkins). • You are free to use, modify, and distribute these slides according to the terms of the Creative Commons license (e.g., you must attribute the slides, no commercial uses ar ...
Powerpoint
... walls of the alveoli break down and the alveoli become enlarged. The enlarged alveoli trap the air, making it more difficult to breathe out. ...
... walls of the alveoli break down and the alveoli become enlarged. The enlarged alveoli trap the air, making it more difficult to breathe out. ...
Degree of reduction
... Fats serve as polymeric biological fuel storage. In addition, lipids constitute portions of more complex molecules, such as lipopolysaccharides. ...
... Fats serve as polymeric biological fuel storage. In addition, lipids constitute portions of more complex molecules, such as lipopolysaccharides. ...
Advanced Cellular Respiration Worksheet
... 6. How many carbon dioxide molecules (CO2) are generated per pyruvate in the transition reaction? in the citric acid cycle? So therefore how many CO2 are produced per glucose? 7. How many NADH molecules are generated per glucose in a. glycolysis b. transition reaction ...
... 6. How many carbon dioxide molecules (CO2) are generated per pyruvate in the transition reaction? in the citric acid cycle? So therefore how many CO2 are produced per glucose? 7. How many NADH molecules are generated per glucose in a. glycolysis b. transition reaction ...
A2 revision
... that some of the main terms used in the answer – e.g. thylakoid lumen, stroma, ATP synthetase – are present in Figure 3, so remember to use them to ensure you get all the available marks! Referring to the figure also demonstrates that you are using it as requested in the question. ...
... that some of the main terms used in the answer – e.g. thylakoid lumen, stroma, ATP synthetase – are present in Figure 3, so remember to use them to ensure you get all the available marks! Referring to the figure also demonstrates that you are using it as requested in the question. ...
The Representative Elements: Group 5A Through 8A
... Biological nitrogen fixation requires a complex set of enzymes and a huge expenditure of ATP. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds. Industrial Fixation Under great pressure, at a temperature of 300 ...
... Biological nitrogen fixation requires a complex set of enzymes and a huge expenditure of ATP. Although the first stable product of the process is ammonia, this is quickly incorporated into protein and other organic nitrogen compounds. Industrial Fixation Under great pressure, at a temperature of 300 ...
New substances are formed by chemical reactions. When elements
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
... Compounds formed from non-metals consist of molecules. The atoms in a molecule are joined together by covalent bonds. These bonds form when atoms share pairs of electrons. Chemical formulas The chemical formula of a compound shows how many of each type of atom join together to make the units which m ...
B-Metabolism of Sulphur containing amino acids
... factors like increased cholesterol/ TG / LDL, obesity, hypertension etc. are due to increased level of homocysteine in the blood. Individuals with the highest homocysteine levels have three times the risk of precipitating heart attack, even if all other risk factors are under control, the reason sti ...
... factors like increased cholesterol/ TG / LDL, obesity, hypertension etc. are due to increased level of homocysteine in the blood. Individuals with the highest homocysteine levels have three times the risk of precipitating heart attack, even if all other risk factors are under control, the reason sti ...
Balance this equation:
... How many moles of oxygen, O2, can be produced from two moles of KClO3? 2 KClO3 Æ 2 KCl + 3 O2 ...
... How many moles of oxygen, O2, can be produced from two moles of KClO3? 2 KClO3 Æ 2 KCl + 3 O2 ...
I. Introduction
... 1. The larynx is ____________________________________________________ __________________________________________________________________ 2. The functions of the larynx are ______________________________________ __________________________________________________________________ 3. The larynx is compo ...
... 1. The larynx is ____________________________________________________ __________________________________________________________________ 2. The functions of the larynx are ______________________________________ __________________________________________________________________ 3. The larynx is compo ...
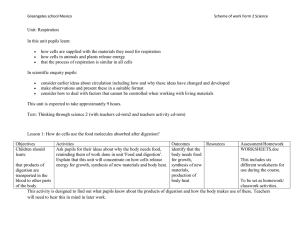
Unit 2 form 2 Respiration scheme of work
... simulation software, that oxygen enters the blood and is where oxygen from the air enters the blood transported elsewhere, and that carbon dioxide produced in the and carbon dioxide in cells passes out of the blood. the blood passes into the alveoli Show illustrations, models or animated pictures of ...
... simulation software, that oxygen enters the blood and is where oxygen from the air enters the blood transported elsewhere, and that carbon dioxide produced in the and carbon dioxide in cells passes out of the blood. the blood passes into the alveoli Show illustrations, models or animated pictures of ...
ch8 - Otterville R-VI School District
... represents identities and relative amounts of reactants and products in the chemical reaction uses symbols and formulas ...
... represents identities and relative amounts of reactants and products in the chemical reaction uses symbols and formulas ...
Name Date
... 9. Fermentation produces no more ATP beyond the small yield from glycolysis, but the remaining reactions a. regenerate ADP c. dump electrons on an inorganic substance (not oxygen) b. regenerate NAD+ d. generate water 10. In certain organisms & under certain conditions, ________ can be used as an ene ...
... 9. Fermentation produces no more ATP beyond the small yield from glycolysis, but the remaining reactions a. regenerate ADP c. dump electrons on an inorganic substance (not oxygen) b. regenerate NAD+ d. generate water 10. In certain organisms & under certain conditions, ________ can be used as an ene ...