Core Concept Cheat Sheet
... range of a few thousands to many millions. ! Functional group: The specific atom or group of atoms that confers a particular chemical property on a biomolecule. ! Organic Compounds: Molecules containing covalently bonded carbon backbones are called organic compounds. ! Hydrolysis: Cleavage of a bond ...
... range of a few thousands to many millions. ! Functional group: The specific atom or group of atoms that confers a particular chemical property on a biomolecule. ! Organic Compounds: Molecules containing covalently bonded carbon backbones are called organic compounds. ! Hydrolysis: Cleavage of a bond ...
Enzymes
... Enzymes act on specific molecules or sets of molecules called substrates They fit into an area of the enzyme called an active site. ...
... Enzymes act on specific molecules or sets of molecules called substrates They fit into an area of the enzyme called an active site. ...
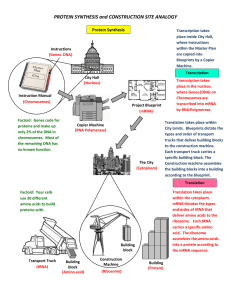
PROTEIN SYNTHESIS and CONSTRUCTION SITE ANALOGY
... Transcription takes place in the nucleus, where Genes (DNA) on Chromosomes are transcribed into mRNA by RNA Polymerase. Translation takes place within City Limits. Blueprints dictate the types and order of transport trucks that deliver building blocks to the construction machine. Each transport truc ...
... Transcription takes place in the nucleus, where Genes (DNA) on Chromosomes are transcribed into mRNA by RNA Polymerase. Translation takes place within City Limits. Blueprints dictate the types and order of transport trucks that deliver building blocks to the construction machine. Each transport truc ...
Oxidation-Reduction (Redox) Reactions
... What happens if solid zinc is placed in a solution containing copper ions? In this case, one species was able to be oxidized while the other was able to be reduced It’s important to have both species present; otherwise where would the electrons come from? Let’s look at the equations again: ...
... What happens if solid zinc is placed in a solution containing copper ions? In this case, one species was able to be oxidized while the other was able to be reduced It’s important to have both species present; otherwise where would the electrons come from? Let’s look at the equations again: ...
CHM121 Exam I Review
... factors, periodic table (periods/groups), chemical formula, balancing equations, nomenclature, molecular/formula weight, percent composition (mass percent), stoichiometry. Be able to define the following terms: states of matter, element, compound, ionic vs. molecular compounds, atom, atomic symbol, ...
... factors, periodic table (periods/groups), chemical formula, balancing equations, nomenclature, molecular/formula weight, percent composition (mass percent), stoichiometry. Be able to define the following terms: states of matter, element, compound, ionic vs. molecular compounds, atom, atomic symbol, ...
Iron Sulfur Proteins and their Synthetic Analogues: Structure
... and those of the proteins but this is probably attributable to a combination of factors already mentioned. Important 4Fe-4S centre proteins (in so far as most information is available about them) are the 8Fe-8S and HiPIP proteins. The HiPIP is exceptional in more ways than its high redox potential- ...
... and those of the proteins but this is probably attributable to a combination of factors already mentioned. Important 4Fe-4S centre proteins (in so far as most information is available about them) are the 8Fe-8S and HiPIP proteins. The HiPIP is exceptional in more ways than its high redox potential- ...
Molecules of Life Worksheet
... 13. The main difference among amino acids is their ____ group. What is the R-group on glycine? on alanine? 14. Differences in R-groups give different proteins different ______________. 15. How does a dipeptide form? 16. What do you call the covalent bonds that hold amino acids together? 17. Long cha ...
... 13. The main difference among amino acids is their ____ group. What is the R-group on glycine? on alanine? 14. Differences in R-groups give different proteins different ______________. 15. How does a dipeptide form? 16. What do you call the covalent bonds that hold amino acids together? 17. Long cha ...
Quantum properties of atomic
... not the case. Guided by this knowledge, in experiments on gold we have discovered that during the contact breaking process the atoms in the contact form stable chains of single atoms being up to 7 atoms long. Such chains constitute the ultimate one-dimensional metallic nanowires. The mechanism behin ...
... not the case. Guided by this knowledge, in experiments on gold we have discovered that during the contact breaking process the atoms in the contact form stable chains of single atoms being up to 7 atoms long. Such chains constitute the ultimate one-dimensional metallic nanowires. The mechanism behin ...
chapter 1 - College Test bank - get test bank and solution manual
... liquid gasoline is converted to heat and gases. Another constructive example is the burning of coal to heat water into steam, which is then used to turn a turbine and produce electricity. The combustion of coal results in a flame plus other gases. The above two examples are examples of chemical chan ...
... liquid gasoline is converted to heat and gases. Another constructive example is the burning of coal to heat water into steam, which is then used to turn a turbine and produce electricity. The combustion of coal results in a flame plus other gases. The above two examples are examples of chemical chan ...
Chemical Equations
... • If the compound is soluble that means that it will remain as ions in the solution, if it is insoluble then the compound precipitated out of the reaction (it became the precipitate or solid). • 2. If at least one INSOLUBLE product is formed (which means a precipitate will form) the reaction will oc ...
... • If the compound is soluble that means that it will remain as ions in the solution, if it is insoluble then the compound precipitated out of the reaction (it became the precipitate or solid). • 2. If at least one INSOLUBLE product is formed (which means a precipitate will form) the reaction will oc ...
EOC Macromolecules
... One category of organic compounds contains molecules composed of long hydrocarbon chains. The hydrocarbon chains may be saturated or unsaturated. ...
... One category of organic compounds contains molecules composed of long hydrocarbon chains. The hydrocarbon chains may be saturated or unsaturated. ...
Lecture 14_withfigures
... M+OH-(s) + nH2O → M+(aq) + OH-(aq) for metals with more ionic bonds → Base MOH + nH2O ...
... M+OH-(s) + nH2O → M+(aq) + OH-(aq) for metals with more ionic bonds → Base MOH + nH2O ...
Slide 1
... 4. When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
... 4. When electrons in an atom in an excited state fall to lower energy levels, energy is 1. absorbed, only 2. released, only 3. neither released nor absorbed 4. both released and absorbed ...
Gas-forming Reactions
... Loss of the first hydrogen ion is virtually complete. So sulfuric acid is classified as strong. The second hydrogen is more difficult to remove and the bisulfate ion is only partially ionized. The bisulfate ion is a weak acid. A base is a substance that increases the concentration of aqueous OH– ion ...
... Loss of the first hydrogen ion is virtually complete. So sulfuric acid is classified as strong. The second hydrogen is more difficult to remove and the bisulfate ion is only partially ionized. The bisulfate ion is a weak acid. A base is a substance that increases the concentration of aqueous OH– ion ...
Nucleic Acids and Protein Synthesis
... III. DNA and Gene Expression A. Eukaryotic cells (DNA in a nucleus) have regions of DNA that do and do not code for proteins. B. Regions of DNA that code for proteins or traits are ...
... III. DNA and Gene Expression A. Eukaryotic cells (DNA in a nucleus) have regions of DNA that do and do not code for proteins. B. Regions of DNA that code for proteins or traits are ...
Exam1 Fall03
... The Levinthal “golf course” landscape theory of protein folding a. shows that as a protein forms more intrachain contacts, internal energy of the protein decreases with its conformational freedom. b. shows that the protein randomly searches for its native conformation until it reaches a “canyon” in ...
... The Levinthal “golf course” landscape theory of protein folding a. shows that as a protein forms more intrachain contacts, internal energy of the protein decreases with its conformational freedom. b. shows that the protein randomly searches for its native conformation until it reaches a “canyon” in ...
What are reactions? - UTLNET Secure Site
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
... If they are formed in a reaction you will see __________. This can be a sign that a chemical __________ has happened. 2. Other signs of a chemical reaction might be an increase in temperature if _____ is released or a change in ________. 3. Physical changes like _________ do not make new materials a ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.