Biology1FinalExam I F'04.doc
... a. carbon, hydrogen, oxygen and phosphorous. b. carbon, hydrogen, nitrogen and oxygen. c. carbon, oxygen, nitrogen and phosphorous. d. carbon, oxygen, nitrogen and iron. e. carbon, oxygen, nitrogen and calcium. 11. The beta-sheet and alpha-helix of a folded protein/polypeptide would be examples of i ...
... a. carbon, hydrogen, oxygen and phosphorous. b. carbon, hydrogen, nitrogen and oxygen. c. carbon, oxygen, nitrogen and phosphorous. d. carbon, oxygen, nitrogen and iron. e. carbon, oxygen, nitrogen and calcium. 11. The beta-sheet and alpha-helix of a folded protein/polypeptide would be examples of i ...
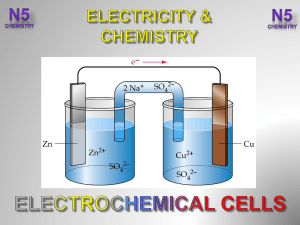
3.-Electrochemical-Cells-V2-
... are batteries which, when they go ‘flat’, can be charged and re-used. This means during recharging the chemicals used in the redox reactions are reformed. The lead-acid battery is the oldest type of rechargeable battery. The battery is made from plates and ...
... are batteries which, when they go ‘flat’, can be charged and re-used. This means during recharging the chemicals used in the redox reactions are reformed. The lead-acid battery is the oldest type of rechargeable battery. The battery is made from plates and ...
Chapter 3 Review Questions
... 20. Proteins will not function properly if they have the wrong __shape__________. 21. An ___enzyme__________ is made of proteins and catalyzes reactions 22. Monomers are linked together by the process of _____polymerization__________. 23. Polymers are broken apart by the process of ___hydrolysis___ ...
... 20. Proteins will not function properly if they have the wrong __shape__________. 21. An ___enzyme__________ is made of proteins and catalyzes reactions 22. Monomers are linked together by the process of _____polymerization__________. 23. Polymers are broken apart by the process of ___hydrolysis___ ...
chapter-23
... 25. There are many biological molecules that contain high-energy phosphate bonds. ATP is used to power life processes because the energy of hydrolysis of ATP is ________. a. intermediate between the energies of hydrolysis of other organophosphate molecules b. small enough that ADP can easily be recy ...
... 25. There are many biological molecules that contain high-energy phosphate bonds. ATP is used to power life processes because the energy of hydrolysis of ATP is ________. a. intermediate between the energies of hydrolysis of other organophosphate molecules b. small enough that ADP can easily be recy ...
protein - The Robinson Group – University of Nottingham
... Peptidyl polymers A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
... Peptidyl polymers A few amino acids in a chain are called a polypeptide. A protein is usually composed of 50 to 400+ amino acids. Since part of the amino acid is lost during dehydration synthesis, we call the units of a protein amino acid residues. carbonyl carbon ...
03 Periodicity
... • Groups - Vertical columns from I to VIII; number of outershell/valence electrons; down a group – gradual change in physical properties and similar chem. properties • Period – Horizontal rows from 1 to 7; number of occupied (electron) shells; ...
... • Groups - Vertical columns from I to VIII; number of outershell/valence electrons; down a group – gradual change in physical properties and similar chem. properties • Period – Horizontal rows from 1 to 7; number of occupied (electron) shells; ...
Semester 2 Review WS
... b.) When hydrochloric acid is added to sodium bicarbonate, it produces water, sodium chloride and carbon dioxide. If 20.0 grams of sodium bicarbonate reacts and 6.75 g of CO2 is produced, what is the percent yield of the carbon dioxide? ...
... b.) When hydrochloric acid is added to sodium bicarbonate, it produces water, sodium chloride and carbon dioxide. If 20.0 grams of sodium bicarbonate reacts and 6.75 g of CO2 is produced, what is the percent yield of the carbon dioxide? ...
MULTIPLE CHOICE. Choose the one alternative that best completes
... 25) All of the compounds that can be synthesized or broken down by chemical reactions inside the body are called A) enzymes. B) nutrients. C) metabolites. D) organic compounds. E) inorganic compounds. ...
... 25) All of the compounds that can be synthesized or broken down by chemical reactions inside the body are called A) enzymes. B) nutrients. C) metabolites. D) organic compounds. E) inorganic compounds. ...
complete week three vocabulary
... Glycolysis-‐ “glucose cutting”; glucose is cut into two pyruvates as a starting step for either cellular respiration or fermentation Kinetic Energy-‐ energy of motion Krebs / Citric Acid Cycle-‐ a cycle ...
... Glycolysis-‐ “glucose cutting”; glucose is cut into two pyruvates as a starting step for either cellular respiration or fermentation Kinetic Energy-‐ energy of motion Krebs / Citric Acid Cycle-‐ a cycle ...
Why teach a course in bioinformatics?
... spontaneously. It takes only a fraction of a second for a floppy chain of beads to fold into the shape it will keep for the rest of its working life. • How does that happen? How do the linear -- and, in some sense, one-dimensional -structures of proteins carry the information that tells them to take ...
... spontaneously. It takes only a fraction of a second for a floppy chain of beads to fold into the shape it will keep for the rest of its working life. • How does that happen? How do the linear -- and, in some sense, one-dimensional -structures of proteins carry the information that tells them to take ...
Organic chemistry
... Biochemistry is the study of the chemical interactions of living things. Biochemists study the structures and physical properties of biological molecules. ...
... Biochemistry is the study of the chemical interactions of living things. Biochemists study the structures and physical properties of biological molecules. ...
Chapter 6 Crossword Puzzle
... Body organ where the majority of proteins are disassembled into amino acids Increased dietary protein intake can lead to increased excretion of the mineral _____. Amino acids can be used to make glucose if insufficient dietary _____ are consumed. What the body uses to assemble its own proteins Prote ...
... Body organ where the majority of proteins are disassembled into amino acids Increased dietary protein intake can lead to increased excretion of the mineral _____. Amino acids can be used to make glucose if insufficient dietary _____ are consumed. What the body uses to assemble its own proteins Prote ...
Review: proteins
... 3. There are _______________ kinds of amino acids, differing from each other only in their ______________ groups. 4. There are _______________ amino acids that humans can't manufacture, these must be obtained from food. They are called _______________ amino acids. 5. Use the following words to descr ...
... 3. There are _______________ kinds of amino acids, differing from each other only in their ______________ groups. 4. There are _______________ amino acids that humans can't manufacture, these must be obtained from food. They are called _______________ amino acids. 5. Use the following words to descr ...
Slide 1
... • Cholesterol - component in animal cell membranes. • Cholesterol – also forms hormones (i.e. testosterone, estrogen) ...
... • Cholesterol - component in animal cell membranes. • Cholesterol – also forms hormones (i.e. testosterone, estrogen) ...
Oregon State University, Summer 2009 Chemistry 121 Midterm
... This exam consists of 20 multiple-choice questions. Each multiple-choice question has 5 points associated with it. Select the best answer by filling in the corresponding circle on the rear page of the answer sheet. If you have any questions before the exam, please ask. If you have any questions duri ...
... This exam consists of 20 multiple-choice questions. Each multiple-choice question has 5 points associated with it. Select the best answer by filling in the corresponding circle on the rear page of the answer sheet. If you have any questions before the exam, please ask. If you have any questions duri ...
The Chemistry of Biology
... 31. Which of the following functional groups is mismatched to the organic compound? A. Phosphate-carbohydrates B. Sulfhydryl-proteins C. Amino-proteins D. Hydroxyl-alcohols E. Carboxyl-fatty acids 32. Organic chemicals always have a basic framework of the element _____ bonded to other atoms. A. Car ...
... 31. Which of the following functional groups is mismatched to the organic compound? A. Phosphate-carbohydrates B. Sulfhydryl-proteins C. Amino-proteins D. Hydroxyl-alcohols E. Carboxyl-fatty acids 32. Organic chemicals always have a basic framework of the element _____ bonded to other atoms. A. Car ...
practice exercise
... Solution Elements that are in the same group of the periodic table are most likely to exhibit similar chemical and physical properties. We therefore expect that Ca and Mg should be most alike because they are in the same group (2A, the alkaline earth metals). ...
... Solution Elements that are in the same group of the periodic table are most likely to exhibit similar chemical and physical properties. We therefore expect that Ca and Mg should be most alike because they are in the same group (2A, the alkaline earth metals). ...
Chapter 18 Review 18.1 Oxidation-Reduction Reactions Oxidation
... where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the electrode where oxidation occurs Cathode- the electrode where reduction occurs Electrolysis- electrical energy is used to produce a chemical change - batteries uses e ...
... where the agents are separated, the electrons flow through a wire, and there is a salt bridge connecting the two solutions Anode- the electrode where oxidation occurs Cathode- the electrode where reduction occurs Electrolysis- electrical energy is used to produce a chemical change - batteries uses e ...
Main concepts Carbohydrates Fats, Proteins and Enzymes
... 2. Carbohydrates are composed of the three elements carbon, hydrogen and oxygen. 3. Carbohydrates include monosaccharides, disaccharides and polysaccharides. 4. The molecular formulas for carbohydrates can be represented as Cx(H2O)y. 5. Monosaccharides form the basis of all other carbohydrates. 6. D ...
... 2. Carbohydrates are composed of the three elements carbon, hydrogen and oxygen. 3. Carbohydrates include monosaccharides, disaccharides and polysaccharides. 4. The molecular formulas for carbohydrates can be represented as Cx(H2O)y. 5. Monosaccharides form the basis of all other carbohydrates. 6. D ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... other dn metal ions under identical ligand framework. Comment upon this observation. 2. It is exceedingly difficult to synthesize low-spin tetrahedral complexes of first row transition metals. Rationalize. 3. In the presence of moderate to strong field ligands Co(II) has a strong tendency to get oxi ...
... other dn metal ions under identical ligand framework. Comment upon this observation. 2. It is exceedingly difficult to synthesize low-spin tetrahedral complexes of first row transition metals. Rationalize. 3. In the presence of moderate to strong field ligands Co(II) has a strong tendency to get oxi ...
Bio A
... concentration level can affect enzyme. Many factors can effect the productivity of an enzyme pH and temperature can both alter the shape of an enzyme, which prevents it from attaching correctly to the substrate Concentration level can effect the reaction by limiting the amount of enzyme availa ...
... concentration level can affect enzyme. Many factors can effect the productivity of an enzyme pH and temperature can both alter the shape of an enzyme, which prevents it from attaching correctly to the substrate Concentration level can effect the reaction by limiting the amount of enzyme availa ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.