TD7: Gel Electrophoresis Photoaffinity probes GEL
... Less efficient but smaller, less invasive Aryl azids: irradiate, loss N2, rapid formation of dehyroazepine imtermediate, which reacts with uncleophiles rather than C-H bonks ...
... Less efficient but smaller, less invasive Aryl azids: irradiate, loss N2, rapid formation of dehyroazepine imtermediate, which reacts with uncleophiles rather than C-H bonks ...
2nd Semester Chemistry Terms - Glancy 4TH PERIOD PHYSICAL
... Electrode- Any material that conducts electrons into or out of a medium in which electrochemical reactions are occurring. ...
... Electrode- Any material that conducts electrons into or out of a medium in which electrochemical reactions are occurring. ...
PowerPoint 1.5MB - The Biomolecular Modeling & Computational
... • Why bother about proteins/prediction • Concepts of molecular modelling – The physicist’s approach – The biologist’s approach ...
... • Why bother about proteins/prediction • Concepts of molecular modelling – The physicist’s approach – The biologist’s approach ...
An Agriscience Lesson Plan: Protein Needs
... • No danger in over feeding protein, but it is usually the most expensive part of the feed • Once the animal has consumed all the protein needed for cell construction, muscle, fetal growth, etc., the rest is broken down for energy • Carbohydrates are a cheaper source of energy ...
... • No danger in over feeding protein, but it is usually the most expensive part of the feed • Once the animal has consumed all the protein needed for cell construction, muscle, fetal growth, etc., the rest is broken down for energy • Carbohydrates are a cheaper source of energy ...
AP Chemistry Summer Assignment
... For those students who have already taken a high school chemistry course, much of the material in the summer packet will be familiar to you. For those students who will be taking AP Chemistry as your first high school chemistry course, the problems will help you build a foundation in chemistry and i ...
... For those students who have already taken a high school chemistry course, much of the material in the summer packet will be familiar to you. For those students who will be taking AP Chemistry as your first high school chemistry course, the problems will help you build a foundation in chemistry and i ...
Fusion, Affinity and Epitope Tags Lecture Notes Handout
... 5, 6 or up to 12 Histadines placed at the terminus of a protein Functional group of Histadine (His) is imidazole ü Negative charged aa ligands with Ni+2 bound to beads ü Binding may improve with amino acid spacers – His-x-x-His-x-x this aligns imidazole ring to same side and allows for flexibility ...
... 5, 6 or up to 12 Histadines placed at the terminus of a protein Functional group of Histadine (His) is imidazole ü Negative charged aa ligands with Ni+2 bound to beads ü Binding may improve with amino acid spacers – His-x-x-His-x-x this aligns imidazole ring to same side and allows for flexibility ...
ap chemistry unit two notes
... Mass conservation illustrated if number of each atom before and after reaction remains constant. Definite composition illustrated by formation of compounds that always have the same atom ratio. Different compounds made of same elements have small whole number ratios of those elements ...
... Mass conservation illustrated if number of each atom before and after reaction remains constant. Definite composition illustrated by formation of compounds that always have the same atom ratio. Different compounds made of same elements have small whole number ratios of those elements ...
PyMOL tutorial
... 5. There are also water molecules around the ion, but they can’t be seen in the sticks representations. To show the water molecules, use: (near_mg)Show->nb_spheres This will show the oxygen atoms of the water molecules as small red spheres 6. To see the identities of the residues neighboring magnesi ...
... 5. There are also water molecules around the ion, but they can’t be seen in the sticks representations. To show the water molecules, use: (near_mg)Show->nb_spheres This will show the oxygen atoms of the water molecules as small red spheres 6. To see the identities of the residues neighboring magnesi ...
The Chemical Level of Organization
... • Buffers convert strong acids to weaker ones which contribute fewer H+ ions & have less effect on pH – carbonic acid - bicarbonate buffer system – together they contribute H+ or OH- ions as needed to keep the pH of the blood stable ...
... • Buffers convert strong acids to weaker ones which contribute fewer H+ ions & have less effect on pH – carbonic acid - bicarbonate buffer system – together they contribute H+ or OH- ions as needed to keep the pH of the blood stable ...
Aminoacids_followup
... Amino acids with hydroxyl group In biology hydroxyl groups –OH are important as they can be modified by different molecules as phosphate (-PO4) or a long range of ...
... Amino acids with hydroxyl group In biology hydroxyl groups –OH are important as they can be modified by different molecules as phosphate (-PO4) or a long range of ...
The receptors have a long extracellular domain
... cyclase which produces cAMP • cAMP activates PKA which phosphorylates voltage gated calcium channel, prolonging ...
... cyclase which produces cAMP • cAMP activates PKA which phosphorylates voltage gated calcium channel, prolonging ...
1 - PBL Group 14
... Channels are generally either cation-selective or anion-selective. Cation-selective channels may be selective for Na+, Ca2+ or K+, or non-selective and permeable to all three. Anion channels are mainly permeable to Cl-, although other types also occur. GATING Voltage-gated channels These channels op ...
... Channels are generally either cation-selective or anion-selective. Cation-selective channels may be selective for Na+, Ca2+ or K+, or non-selective and permeable to all three. Anion channels are mainly permeable to Cl-, although other types also occur. GATING Voltage-gated channels These channels op ...
Ligand Binding - Stroud
... nuclear receptor assembly on DNA direct repeats. 1995 Nature 375, 203-211. • DNA-binding proteins often share common structural motifs • The major groove, minor groove, and backbone provide specific recognition points • Water molecules often are located at protein-nucleic acid interfaces • Oligomeri ...
... nuclear receptor assembly on DNA direct repeats. 1995 Nature 375, 203-211. • DNA-binding proteins often share common structural motifs • The major groove, minor groove, and backbone provide specific recognition points • Water molecules often are located at protein-nucleic acid interfaces • Oligomeri ...
Enzyme promiscuity is an ability to catalyze
... bp which encodes for 299 amino acids long protein. 70-90% of amino acid sequence identity was observed among vertebrates, which is evocative of an essential biological function. It is also reported as Ca2+-binding protein involved in the regulation of free cellular Ca2+ , hence also named as “Re ...
... bp which encodes for 299 amino acids long protein. 70-90% of amino acid sequence identity was observed among vertebrates, which is evocative of an essential biological function. It is also reported as Ca2+-binding protein involved in the regulation of free cellular Ca2+ , hence also named as “Re ...
Elements (NonMetals)
... Gas at room Temp B.P. –253°C (20K) and M.P.-259°C (14K) Insoluble in water: 2mL gas/ 1L of water Found in H2O, organic and biological molecules Most common element in universe H2 (H-H) isoelectronic with He H has a small radius Unique properties of both group 1 and 17 Bond energy 431kJ/mol – very st ...
... Gas at room Temp B.P. –253°C (20K) and M.P.-259°C (14K) Insoluble in water: 2mL gas/ 1L of water Found in H2O, organic and biological molecules Most common element in universe H2 (H-H) isoelectronic with He H has a small radius Unique properties of both group 1 and 17 Bond energy 431kJ/mol – very st ...
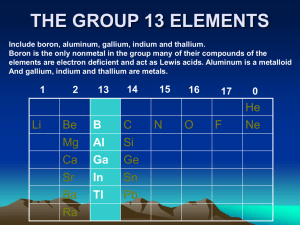
THE GROUP 13 ELEMENTS - University of the Witwatersrand
... Wade’s Rules: established by Kenneth Wade in the 1970s based on correlation between the number of electrons, the formula and the shape of the molecules. This apply to a class of polyhedra called deltahedra because they are made up of triangular faces resembling Δ. For molecular and anionic boranes ...
... Wade’s Rules: established by Kenneth Wade in the 1970s based on correlation between the number of electrons, the formula and the shape of the molecules. This apply to a class of polyhedra called deltahedra because they are made up of triangular faces resembling Δ. For molecular and anionic boranes ...
Anaerobic Respiration
... electron acceptor is reduced and used as the source of nutrient for cell growth. Dissimilative metabolism: A large amount of the electron acceptor is reduced for energy and the reduced product is excreted into the environment. ...
... electron acceptor is reduced and used as the source of nutrient for cell growth. Dissimilative metabolism: A large amount of the electron acceptor is reduced for energy and the reduced product is excreted into the environment. ...
review for characteristics of life/macromolecules/enzymes test
... to 1 of 2 groups. Volunteers in Group 1 are given the medication, and volunteers in Group 2 are given a placebo. Which of the following steps is necessary to ensure the scientific validity of the results? A. ...
... to 1 of 2 groups. Volunteers in Group 1 are given the medication, and volunteers in Group 2 are given a placebo. Which of the following steps is necessary to ensure the scientific validity of the results? A. ...
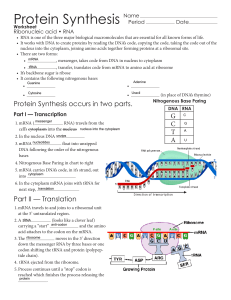
Protein Synthesis - Issaquah Connect
... nucleus into the cytoplasm, joining amino acids together forming proteins at a ribosomal site. • There are two forms: • mRNA , messenger, takes code from DNA in nucleus to cytoplasm • tRNA , transfer, translates code from mRNA to amino acid at ribosome • It’s backbone sugar is ribose • It conta ...
... nucleus into the cytoplasm, joining amino acids together forming proteins at a ribosomal site. • There are two forms: • mRNA , messenger, takes code from DNA in nucleus to cytoplasm • tRNA , transfer, translates code from mRNA to amino acid at ribosome • It’s backbone sugar is ribose • It conta ...
Macromolecules Worksheet #2
... They are isomers of one another – They have the same chemical formula but differ in how those elements are bonded to each other within the molecule. 2. What are the structural differences between a saturated and an unsaturated fat? Unsaturated fats have a double bond between at least two carbons in ...
... They are isomers of one another – They have the same chemical formula but differ in how those elements are bonded to each other within the molecule. 2. What are the structural differences between a saturated and an unsaturated fat? Unsaturated fats have a double bond between at least two carbons in ...
Name: Per: Date: Unit 1. Materials: Formulating Matter B. Periodic
... 38. Fill in the data table for each ionic compound described below. Number one is filled in as an example. Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO ...
... 38. Fill in the data table for each ionic compound described below. Number one is filled in as an example. Use the two tables of common ions below. a. Potassium chloride is “lite salt”, used by many people with hypertension. b. CaSO4 is a component of plaster. c. A substance composed of Ca2+ and PO ...
Biology Content Standards
... of very few elements. The six most common are C, H, #, O, P, S. 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activa ...
... of very few elements. The six most common are C, H, #, O, P, S. 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activa ...
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.