Name
... 5) Compare and Contrast the Light and Dark Reactions with regards to their reactants, products, and their requirements for photons of light or ribulose-1,5-bisphosphate (5 points) The light reactions require light to remove an electron from water to make oxygen in Photosystem II, then use the energy ...
... 5) Compare and Contrast the Light and Dark Reactions with regards to their reactants, products, and their requirements for photons of light or ribulose-1,5-bisphosphate (5 points) The light reactions require light to remove an electron from water to make oxygen in Photosystem II, then use the energy ...
Polymers and Amino Acids
... Globular proteins usually have a spherical shape caused by tightly folded polypeptide chains with hydrophobic groups on the inside, and hydrophilic groups on the outside. ...
... Globular proteins usually have a spherical shape caused by tightly folded polypeptide chains with hydrophobic groups on the inside, and hydrophilic groups on the outside. ...
ESCHERICHIA COLI
... of herbal and recreational drugs as well as medicines. In recent years, the use of some secondary metabolites as an alternative to conventional antibiotics has generated the interest in human health research. More than 13,000 secondary metabolites have been isolated from the medicinal plants which a ...
... of herbal and recreational drugs as well as medicines. In recent years, the use of some secondary metabolites as an alternative to conventional antibiotics has generated the interest in human health research. More than 13,000 secondary metabolites have been isolated from the medicinal plants which a ...
The Nitrogen Cycle
... decomposers deplete water of dissolved oxygen and disrupt aquatic systems and ...
... decomposers deplete water of dissolved oxygen and disrupt aquatic systems and ...
Integrated Microbial Genomes
... complete genomes, currently consists of 2791 COGs including 45 350 proteins from 30 genomes of bacteria, archaea and the yeast Saccharomyces cerevisiae (http://www.ncbi.nlm.nih.gov/COG). In addition, a supplement to the COGs is available, in which proteins encoded in the genomes of two multicellular ...
... complete genomes, currently consists of 2791 COGs including 45 350 proteins from 30 genomes of bacteria, archaea and the yeast Saccharomyces cerevisiae (http://www.ncbi.nlm.nih.gov/COG). In addition, a supplement to the COGs is available, in which proteins encoded in the genomes of two multicellular ...
1 Respiration efficiency Respiration summary
... – Phosphorylation of a single serine residue in the phosphorylase enzyme (a covalent post-translational modification) turns off glycogen to glucose conversion. ...
... – Phosphorylation of a single serine residue in the phosphorylase enzyme (a covalent post-translational modification) turns off glycogen to glucose conversion. ...
Vll. Nitrogen metabolism:
... • Humans can synthesize 11 of 20 amino acids • others are essential in the diet • Amino acid metabolism uses cofactors PLP, others • Dietary nonessential aa made from glycolytic intermediates or from existing aa • Amino acids are degraded to urea; Carbon skeleton is glucogenic or ketogenic • Defects ...
... • Humans can synthesize 11 of 20 amino acids • others are essential in the diet • Amino acid metabolism uses cofactors PLP, others • Dietary nonessential aa made from glycolytic intermediates or from existing aa • Amino acids are degraded to urea; Carbon skeleton is glucogenic or ketogenic • Defects ...
Lehninger Principles of Biochemistry 5/e
... Linear arrangement of n amino acid residues linked by peptide bonds. Polymers composed of two, three, a few, and many amino acid residues are called as dipeptides, tripeptides, oligopeptides and polypeptides. Proteins are molecules that consist of one or more polypeptide chains. ...
... Linear arrangement of n amino acid residues linked by peptide bonds. Polymers composed of two, three, a few, and many amino acid residues are called as dipeptides, tripeptides, oligopeptides and polypeptides. Proteins are molecules that consist of one or more polypeptide chains. ...
Cellular Respiration
... Nutrients + oxygen water + ATP + CO2 • Changes the chemical energy in glucose into the chemical energy in ATP ...
... Nutrients + oxygen water + ATP + CO2 • Changes the chemical energy in glucose into the chemical energy in ATP ...
Chemistry Chapter 9.1 Making Predictions About Solubility
... Problem: Aqueous solutions that contain silver ions are usually treated with chloride ions to recover silver chloride. What is the minimum volume of 0.25mol/L magnesium chloride needed to precipitate all silver ions in 60mL of ...
... Problem: Aqueous solutions that contain silver ions are usually treated with chloride ions to recover silver chloride. What is the minimum volume of 0.25mol/L magnesium chloride needed to precipitate all silver ions in 60mL of ...
here
... Read Chapter 9 in Campbell & Reece. Answer the following questions. Glycolysis & Krebs Cycle HW 1. Contrast glycolysis with cell respiration, citing such factors as locale, oxygen use, energy yields, and type of phosphorylation used. 2. Briefly describe the two means by which ADP is phosphorylated. ...
... Read Chapter 9 in Campbell & Reece. Answer the following questions. Glycolysis & Krebs Cycle HW 1. Contrast glycolysis with cell respiration, citing such factors as locale, oxygen use, energy yields, and type of phosphorylation used. 2. Briefly describe the two means by which ADP is phosphorylated. ...
Key area 2 * Cellular respiration
... • How many ATP are produced during aerobic respiration? • How many ATP are produced during anaerobic respiration? • Which is more efficient? ...
... • How many ATP are produced during aerobic respiration? • How many ATP are produced during anaerobic respiration? • Which is more efficient? ...
Fermentation - mvhs
... Fermentation • Occurs when there is no oxygen available • allows some cells to produce ATP without the use of oxygen – ATP yield would be lower, though. Do you know why? – Only glycolysis is carried out– only 2 ATP produced. ...
... Fermentation • Occurs when there is no oxygen available • allows some cells to produce ATP without the use of oxygen – ATP yield would be lower, though. Do you know why? – Only glycolysis is carried out– only 2 ATP produced. ...
11.Chemical elements and their classification. S
... 3. The sodium bicarbonate (NaHCO3) that precipitates out in reaction (I) is filtered out from the hot NH4Cl solution, and the solution is then reacted with the CaO left over from heating the limestone in step (II). 2 NH4Cl + CaO → 2 NH3 + CaCl2 + H2O (III) CaO makes a strong basic solution. The NH3 ...
... 3. The sodium bicarbonate (NaHCO3) that precipitates out in reaction (I) is filtered out from the hot NH4Cl solution, and the solution is then reacted with the CaO left over from heating the limestone in step (II). 2 NH4Cl + CaO → 2 NH3 + CaCl2 + H2O (III) CaO makes a strong basic solution. The NH3 ...
Group 6
... In the egg whites the albumin will change from clear to white. We will explore how the following denature egg albumin as well as milk casein. When egg white is heated above a certain temperature (i.e. 60 °C) bonds in the protein molecules break and reform in different ways. This causes the protein m ...
... In the egg whites the albumin will change from clear to white. We will explore how the following denature egg albumin as well as milk casein. When egg white is heated above a certain temperature (i.e. 60 °C) bonds in the protein molecules break and reform in different ways. This causes the protein m ...
Balancing Chemical Equations – A Primer
... Another change...ions with more than one ionic charge Some ions have more than one charge. For example, copper is either Cu1+ or Cu2+, lead is Pb2+ or Pb4+ and iron is Fe2+ and Fe 3+ Which one do you use? The clue is in the written name of the compound. The compound name will include a ROMAN NUMERAL ...
... Another change...ions with more than one ionic charge Some ions have more than one charge. For example, copper is either Cu1+ or Cu2+, lead is Pb2+ or Pb4+ and iron is Fe2+ and Fe 3+ Which one do you use? The clue is in the written name of the compound. The compound name will include a ROMAN NUMERAL ...
File
... 5. The ionic bond is weak in the presence of water. The hydrogen bond is also weak but crucial to life. L. Hydrogen Bonds/Van der Walls Interactions 1. A hydrogen bond occurs when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom. 2. In l ...
... 5. The ionic bond is weak in the presence of water. The hydrogen bond is also weak but crucial to life. L. Hydrogen Bonds/Van der Walls Interactions 1. A hydrogen bond occurs when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom. 2. In l ...
file
... • Biological catalysts – proteins (& RNA) – facilitate chemical reactions • increase rate of reaction without being consumed • reduce activation energy • don’t change free energy (G) released or required ...
... • Biological catalysts – proteins (& RNA) – facilitate chemical reactions • increase rate of reaction without being consumed • reduce activation energy • don’t change free energy (G) released or required ...
updated ppt slides - UCLA Chemistry and Biochemistry
... The symmetry (concerted) model of cooperativity Subunits can adopt one of two possible conformations: T or R. All subunits must adopt the same conformation (protein is always symmetric). Equilibrium between T and R states is influenced by ligand or modulator binding. The sequential (gradual) model ...
... The symmetry (concerted) model of cooperativity Subunits can adopt one of two possible conformations: T or R. All subunits must adopt the same conformation (protein is always symmetric). Equilibrium between T and R states is influenced by ligand or modulator binding. The sequential (gradual) model ...
8.1 Condensation Polymers
... Each monomer in a condensation polymer must have at least two functional groups. LO: I know the structure of starch. ...
... Each monomer in a condensation polymer must have at least two functional groups. LO: I know the structure of starch. ...
De Novo Sequencing and Homology Search with De Novo
... employ a two-stage approach. – First, compute many (e.g. 10,000) sequences using an efficient score function that uses only a few of the most important ions. – Then, evaluate these candidates using a more sophisticated scoring function additional ions. ...
... employ a two-stage approach. – First, compute many (e.g. 10,000) sequences using an efficient score function that uses only a few of the most important ions. – Then, evaluate these candidates using a more sophisticated scoring function additional ions. ...
Classification of Enzymes
... Exercises • 7. Which of the following statements is false? a) A reaction may not occur at a detectable rate even though it has a favorable equilibrium. b) After a reaction, the enzyme involved becomes available to catalyze the reaction again. c) For S P, a catalyst shifts the reaction equilibrium t ...
... Exercises • 7. Which of the following statements is false? a) A reaction may not occur at a detectable rate even though it has a favorable equilibrium. b) After a reaction, the enzyme involved becomes available to catalyze the reaction again. c) For S P, a catalyst shifts the reaction equilibrium t ...
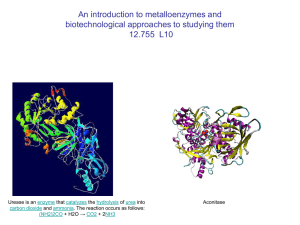
Metalloprotein

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large number of all proteins are part of this category.