lesson-13-protein-denaturation-handout
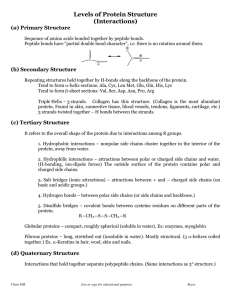
... The three-dimensional conformation of proteins is stabilized by bonds or interactions between R groups of amino acids within the molecule. Most of these bonds and interactions are relatively weak and they can be disrupted or broken. This results in a change to the conformation of the protein, which ...
... The three-dimensional conformation of proteins is stabilized by bonds or interactions between R groups of amino acids within the molecule. Most of these bonds and interactions are relatively weak and they can be disrupted or broken. This results in a change to the conformation of the protein, which ...
How to start to crystallise proteins
... common usage. This has resulted in a number of minimal screens being proposed. For the beginner, then, it may be prudent and worth trying such a minimal screen in the hope of a lucky result, especially as this can involve as few as 24 trial conditions. At the JCSG in Toronto it was found that out of ...
... common usage. This has resulted in a number of minimal screens being proposed. For the beginner, then, it may be prudent and worth trying such a minimal screen in the hope of a lucky result, especially as this can involve as few as 24 trial conditions. At the JCSG in Toronto it was found that out of ...
Chem 400 Biochemistry I
... Sephacryl S-200 has a fractionation range of 5 kDa to 250 kDa What is the exclusion limit? – Would this be appropriate for a set of proteins with molecular weights of 8 kDa, 15 kDa, 200 kDa and 500 kDa? ...
... Sephacryl S-200 has a fractionation range of 5 kDa to 250 kDa What is the exclusion limit? – Would this be appropriate for a set of proteins with molecular weights of 8 kDa, 15 kDa, 200 kDa and 500 kDa? ...
BIO Ques Bank protein - Vishwa Bharti Public School
... Name any four diseases caused due to absence / alteration of key proteins. Functional properties of proteins depend upon their 3D structures. Explain with a suitable example. Which of the two is a stronger bond and why? (a) Ionic or covalent bond in vacuum (b) Ionic or covalent bond in H2O When oil ...
... Name any four diseases caused due to absence / alteration of key proteins. Functional properties of proteins depend upon their 3D structures. Explain with a suitable example. Which of the two is a stronger bond and why? (a) Ionic or covalent bond in vacuum (b) Ionic or covalent bond in H2O When oil ...
week 5 no answers
... b. Due to a very high concentration of proteins in solution. 2 classes of molecular chaperones. ...
... b. Due to a very high concentration of proteins in solution. 2 classes of molecular chaperones. ...
Tertiary Structure to X-Ray Crystallography
... very middle of the structure of 3EWC is a bound molecule, visible in the center of the structure. This bound molecule blocks of the function of adenosine deaminase and inhibits its function. Since tertiary structure describes the three-dimensional shape of a protein, another level of protein structu ...
... very middle of the structure of 3EWC is a bound molecule, visible in the center of the structure. This bound molecule blocks of the function of adenosine deaminase and inhibits its function. Since tertiary structure describes the three-dimensional shape of a protein, another level of protein structu ...
Document
... Biological properties of proteins result from interactions with other molecules Antibodies, enzymes, structure, etc ...
... Biological properties of proteins result from interactions with other molecules Antibodies, enzymes, structure, etc ...
Surface-active ionic liquids applied on the recovery of green
... spectral and fluorescence characteristics. As the recombinant GFP is usually expressed intracellularly, for example, by recombinant strains of Escherichia coli [1], a preliminary step of cell disruption is mandatory. The conventional methods of cell disruption include the mechanical methods (e.g., m ...
... spectral and fluorescence characteristics. As the recombinant GFP is usually expressed intracellularly, for example, by recombinant strains of Escherichia coli [1], a preliminary step of cell disruption is mandatory. The conventional methods of cell disruption include the mechanical methods (e.g., m ...
Proteins and The Cell Membrane
... metabolism. Cholera bacteria release a toxin that interferes with the functioning of this enzyme resulting in Na+ and water leaving intestinal cells. Individuals may die from severe diarrhea. ...
... metabolism. Cholera bacteria release a toxin that interferes with the functioning of this enzyme resulting in Na+ and water leaving intestinal cells. Individuals may die from severe diarrhea. ...
The Renal Diet - Pro t e i n
... diet your physician will ask you to follow will be based upon your level of kidney function, your body size, and any other medical conditions you may have. Your diet may be helpful in delaying the need for dialysis. ...
... diet your physician will ask you to follow will be based upon your level of kidney function, your body size, and any other medical conditions you may have. Your diet may be helpful in delaying the need for dialysis. ...
Proteins are biopolymers construced from similar building blocks
... unique feature is that these polypeptide chains are folded in a certain three-dimensional structure (called native structure), which enables them to perform their biological funtion. Studies on protein structure and function by wide range of experimental techniques agree in the fact that the native ...
... unique feature is that these polypeptide chains are folded in a certain three-dimensional structure (called native structure), which enables them to perform their biological funtion. Studies on protein structure and function by wide range of experimental techniques agree in the fact that the native ...
Protein Degradation at Lysosome
... • Cells are continually building proteins, using them for a single task, and then discarding them. • Signaling or controlling proteins (eg. transcription regulators and the cyclins) - lead very brief lives, carrying their messages and then being thrown away. • Specialized enzymes - built just when t ...
... • Cells are continually building proteins, using them for a single task, and then discarding them. • Signaling or controlling proteins (eg. transcription regulators and the cyclins) - lead very brief lives, carrying their messages and then being thrown away. • Specialized enzymes - built just when t ...
Interactive Software for the study of membrane biology: lipid
... characterization of these biomimetic systems using experimental examples. The topics explained in each section were: (1) Membranes composition; lipids and proteins distribution; fluid mosaic model; the basic structural unit of lipid bilayer; peripheral proteins; anchored proteins; integral proteins; ...
... characterization of these biomimetic systems using experimental examples. The topics explained in each section were: (1) Membranes composition; lipids and proteins distribution; fluid mosaic model; the basic structural unit of lipid bilayer; peripheral proteins; anchored proteins; integral proteins; ...
Structural and functional relationship of EBF1 variants in B
... mechanisms of an immensely complex process, including expression of key genes, interaction of transcription factors and activation/deactivation of signaling pathways. Any disturbance of these networks can lead for example to leukemia. High prevalence of early B-cell factor 1 (Ebf1) genetic lesion in ...
... mechanisms of an immensely complex process, including expression of key genes, interaction of transcription factors and activation/deactivation of signaling pathways. Any disturbance of these networks can lead for example to leukemia. High prevalence of early B-cell factor 1 (Ebf1) genetic lesion in ...
Protein Structure 2 - Interactions - Hydrolysis
... 2. Hydrophilic interactions – attractions between polar or charged side chains and water. (H-bonding, ion-dipole forces) The outside surface of the protein contains polar and charged side chains. 3. Salt bridges (ionic attractions) – attractions between + and – charged side chains (on basic and acid ...
... 2. Hydrophilic interactions – attractions between polar or charged side chains and water. (H-bonding, ion-dipole forces) The outside surface of the protein contains polar and charged side chains. 3. Salt bridges (ionic attractions) – attractions between + and – charged side chains (on basic and acid ...
Fundamentals of Biochemistry
... affect a protein’s stability during purification. • An assay based on a protein’s chemical or binding properties may be used to quantify a protein during purification. • Fractionation procedures take advantage of a protein’s unique structure and chemistry in order to separate it from other molecules ...
... affect a protein’s stability during purification. • An assay based on a protein’s chemical or binding properties may be used to quantify a protein during purification. • Fractionation procedures take advantage of a protein’s unique structure and chemistry in order to separate it from other molecules ...
Protein - Canon-MacFCS
... Create and regulate hormones: proteins are one component of hormones that help regulate the systems of the body including blood sugar and metabolism. Create antibodies: proteins made by the body are necessary to destroy foreign substances and prevent illness. Energy source: last choice of energy ...
... Create and regulate hormones: proteins are one component of hormones that help regulate the systems of the body including blood sugar and metabolism. Create antibodies: proteins made by the body are necessary to destroy foreign substances and prevent illness. Energy source: last choice of energy ...
Bioc 462a Lecture Notes
... o In addition, the SDS causes all proteins to adopt a random-coil structure, which means that shape does not effect movement through the gel. o Thus SDS-PAGE is very useful method for determining the molecular weight of a protein. Western blotting is a technique for detecting a specific protein in a ...
... o In addition, the SDS causes all proteins to adopt a random-coil structure, which means that shape does not effect movement through the gel. o Thus SDS-PAGE is very useful method for determining the molecular weight of a protein. Western blotting is a technique for detecting a specific protein in a ...
TD7: Gel Electrophoresis Photoaffinity probes GEL
... TD7: Gel Electrophoresis Photoaffinity probes GEL ELECTROPHORESIS ...
... TD7: Gel Electrophoresis Photoaffinity probes GEL ELECTROPHORESIS ...
RISE-Workshop
... point, pI, at which it is immobile in an electric field. If a mixture of proteins is electrophoresed through a solution or gel that has a stable pH gradient in which the pH smoothly increases from anode to cathode, each protein will migrate to the position in the pH gradient corresponding to its pI. ...
... point, pI, at which it is immobile in an electric field. If a mixture of proteins is electrophoresed through a solution or gel that has a stable pH gradient in which the pH smoothly increases from anode to cathode, each protein will migrate to the position in the pH gradient corresponding to its pI. ...
Highligh in Physics 2005
... wavelength. The dark state corresponds to an absorption peak at 3.5 eV. To compute absorption we use the Time Dependent Density Functional Theory. ...
... wavelength. The dark state corresponds to an absorption peak at 3.5 eV. To compute absorption we use the Time Dependent Density Functional Theory. ...
MCB100A/CHEM130A In-Section Quiz #2 (Aathavan Karunakaran)
... When similar traits develop independently in two different species whose different ancestors didn’t have that trait. ...
... When similar traits develop independently in two different species whose different ancestors didn’t have that trait. ...
Quality Control of Intact Recombinant Proteins Using Sensitive High
... industry as these proteins are increasingly used as drugs. With this interest in new biopharmaceuticals proper quality control is needed to ensure the use of the right batches in the proteins production. This includes knowledge about the correct amino acid sequence as well as characterization of mod ...
... industry as these proteins are increasingly used as drugs. With this interest in new biopharmaceuticals proper quality control is needed to ensure the use of the right batches in the proteins production. This includes knowledge about the correct amino acid sequence as well as characterization of mod ...
The molecular architecture, macro-organization and functions of the
... stabilization of the ultrastructure of thylakoid membranes and in their reorganizations. In addition, these proteins play key roles in important regulatory mechanisms: in excess light, via controlled dissipation governed by low lumenal pH, they are capable of transiently downregulating their light-h ...
... stabilization of the ultrastructure of thylakoid membranes and in their reorganizations. In addition, these proteins play key roles in important regulatory mechanisms: in excess light, via controlled dissipation governed by low lumenal pH, they are capable of transiently downregulating their light-h ...
Protein purification
Protein purification is a series of processes intended to isolate one or a few proteins from a complex mixture, usually cells, tissues or whole organisms. Protein purification is vital for the characterization of the function, structure and interactions of the protein of interest. The purification process may separate the protein and non-protein parts of the mixture, and finally separate the desired protein from all other proteins. Separation of one protein from all others is typically the most laborious aspect of protein purification. Separation steps usually exploit differences in protein size, physico-chemical properties, binding affinity and biological activity. The pure result may be termed protein isolate.The methods used in protein purification can roughly be divided into analytical and preparative methods. The distinction is not exact, but the deciding factor is the amount of protein that can practically be purified with that method. Analytical methods aim to detect and identify a protein in a mixture, whereas preparative methods aim to produce large quantities of the protein for other purposes, such as structural biology or industrial use. In general, the preparative methods can be used in analytical applications, but not the other way around.