Direct production of hydrogen peroxide from CO, O2, and H2O over
... CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versatile hydrogenation applications.11,12 All the results presented ...
... CO/O2/H2O over several types of metal nanoparticles dispersed on alumina prepared by the wet reduction (WR) method, which has recently been shown to be an effective method for the preparation of various amorphous alloy catalysts for versatile hydrogenation applications.11,12 All the results presented ...
Organic Reactions Worksheet
... iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using any secondary alcohol: i) Give the displayed (structural formula) which it could be oxidized to ii) State which homologous series the products are part of iii) Write the balanced equation for each oxidizing reac ...
... iv) Write the balanced equation for each oxidizing reaction, use [O] convention c. Using any secondary alcohol: i) Give the displayed (structural formula) which it could be oxidized to ii) State which homologous series the products are part of iii) Write the balanced equation for each oxidizing reac ...
Learning Guide for Chapter 16
... Synthesis using epoxides What kinds of products can be made using epoxides? compounds with an alcohol next to something that can be attached using a Nu N HO ...
... Synthesis using epoxides What kinds of products can be made using epoxides? compounds with an alcohol next to something that can be attached using a Nu N HO ...
Project Overview
... These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the ...
... These PowerPoint Lecture Slides were created and prepared by Professor William Tam and his wife, Dr. Phillis Chang. Professor William Tam received his B.Sc. at the University of Hong Kong in 1990 and his Ph.D. at the University of Toronto (Canada) in 1995. He was an NSERC postdoctoral fellow at the ...
Details
... Transformation properties of atomic orbitals, Hybridization scheme for σ-bonding (C3v, C4V, D3h, Td) projection operator, Symmetry adopted LCO, Hybrid orbital as linear combination of atomic orbitals, MO treatment of coordination compounds, σ-bonding in octahedral complexes, Formation of LCO, Format ...
... Transformation properties of atomic orbitals, Hybridization scheme for σ-bonding (C3v, C4V, D3h, Td) projection operator, Symmetry adopted LCO, Hybrid orbital as linear combination of atomic orbitals, MO treatment of coordination compounds, σ-bonding in octahedral complexes, Formation of LCO, Format ...
Practical and selective aerobic oxidation of alcohols to
... homogeneous Pd-catalysed aerobic oxidation of alcohols was also reported.10 These systems are very effective, but either require specialised equipment to generate and contain supercritical CO2, or require extensive workup (product This journal is © The Royal Society of Chemistry [year] ...
... homogeneous Pd-catalysed aerobic oxidation of alcohols was also reported.10 These systems are very effective, but either require specialised equipment to generate and contain supercritical CO2, or require extensive workup (product This journal is © The Royal Society of Chemistry [year] ...
CHEM1102 2014-J-8 June 2014 • Complete the following table
... • Methylphenidate, also known as Ritalin, is a psychostimulant drug approved for the treatment of attention-deficit disorder. Identify all stereogenic (chiral) centres in methylphenidate by clearly marking each with an asterisk (*) on the structure below. ...
... • Methylphenidate, also known as Ritalin, is a psychostimulant drug approved for the treatment of attention-deficit disorder. Identify all stereogenic (chiral) centres in methylphenidate by clearly marking each with an asterisk (*) on the structure below. ...
Carbonyl Compounds
... ethanamide has the highest boiling point because it has the potential t form multiple H-bonds between molecules, all the bonds must be broken before the compound can pass to vapour phase. ethanoic acid, however, has fewer H-bonds compared with ethanamide. Propanone has no possibility forming H-bonds ...
... ethanamide has the highest boiling point because it has the potential t form multiple H-bonds between molecules, all the bonds must be broken before the compound can pass to vapour phase. ethanoic acid, however, has fewer H-bonds compared with ethanamide. Propanone has no possibility forming H-bonds ...
Aqueous oxidation of alcohols catalyzed by artificial
... the stoichiometric metal oxidants obsolete. For this purpose, the use of tert-butylhydroperoxide (TBHP) as an oxidizing agent has been investigated in conjunction with several homogeneous transition-metal catalysts, including chromium, ruthenium, rhodium, and copper [9–13]. The present Communication ...
... the stoichiometric metal oxidants obsolete. For this purpose, the use of tert-butylhydroperoxide (TBHP) as an oxidizing agent has been investigated in conjunction with several homogeneous transition-metal catalysts, including chromium, ruthenium, rhodium, and copper [9–13]. The present Communication ...
Resolution of Diols via Catalytic Asymmetric Acetalization
... encouraged us to also explore the kinetic resolution of dialkylsubstituted diols. Gratifyingly, the reaction of methyl,cyclohexylsubstituted diol rac-2o proceeded with a selectivity factor of 23 (entry 9). With methyl,hexyl-substituted diol, the reaction still proceeded with a reasonable selectivity ...
... encouraged us to also explore the kinetic resolution of dialkylsubstituted diols. Gratifyingly, the reaction of methyl,cyclohexylsubstituted diol rac-2o proceeded with a selectivity factor of 23 (entry 9). With methyl,hexyl-substituted diol, the reaction still proceeded with a reasonable selectivity ...
Organic Chemistry
... but different spatial arrangement. • Enantiomers – non-superimposable isomers with identical mirror images (S & R types). • They appear identical, but are chemically distinct. ...
... but different spatial arrangement. • Enantiomers – non-superimposable isomers with identical mirror images (S & R types). • They appear identical, but are chemically distinct. ...
Substrate scope of the re-engineered enzyme, FucO D93
... Enzymes are catalytically active proteins, they are not the only catalysts within living things but by far the most common. The structural diversity of enzymes allow them to catalyse a myriad of reactions as varied as the fixation of atmospheric nitrogen into ammonia and the decomposition of sugar t ...
... Enzymes are catalytically active proteins, they are not the only catalysts within living things but by far the most common. The structural diversity of enzymes allow them to catalyse a myriad of reactions as varied as the fixation of atmospheric nitrogen into ammonia and the decomposition of sugar t ...
Regiospecificity according to Markovnikov
... An Introduction to Organic Synthesis • Organic synthesis creates molecules by design ...
... An Introduction to Organic Synthesis • Organic synthesis creates molecules by design ...
protecting groups
... • Alcohols are most commonly protected as ethers, especially where the ether function is in reality part of a (mixed) acetal or ketal; this enables ...
... • Alcohols are most commonly protected as ethers, especially where the ether function is in reality part of a (mixed) acetal or ketal; this enables ...
Carbon Bond - Rutgers Chemistry
... industrial and synthetic organic chemical processes. Our initial finding of C-H oxidative addition reactions in low-valent iridium (I) complexes was followed by a more recent discovery and exploration of C-H activation processes at Ir (III) centers. This paper reviews recent studies of the Ir (III) ...
... industrial and synthetic organic chemical processes. Our initial finding of C-H oxidative addition reactions in low-valent iridium (I) complexes was followed by a more recent discovery and exploration of C-H activation processes at Ir (III) centers. This paper reviews recent studies of the Ir (III) ...
t.h.e_2 - Homework Market
... 1,3-diaxial (OCH3 and H) 1,3-diaxial (CH3 and t-butyl) 1,3-diaxial (OCH3 and CH3) ...
... 1,3-diaxial (OCH3 and H) 1,3-diaxial (CH3 and t-butyl) 1,3-diaxial (OCH3 and CH3) ...
Principles of Drug Action I, Spring 2004
... on the carbon atom adjacent to that carbon bound to Br. In this case there are two such carbons, but the "interior" one will lose a proton faster because it gives the more stable alkene (more substituted), and generates and alkene where the C=C is in conjugation with the aromatic ring!!!! CH3 ...
... on the carbon atom adjacent to that carbon bound to Br. In this case there are two such carbons, but the "interior" one will lose a proton faster because it gives the more stable alkene (more substituted), and generates and alkene where the C=C is in conjugation with the aromatic ring!!!! CH3 ...
Recall
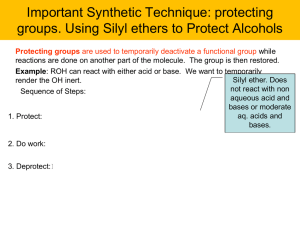
... Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily Silyl ether. Does render the OH inert. not react with non Sequence of ...
... Protecting groups are used to temporarily deactivate a functional group while reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily Silyl ether. Does render the OH inert. not react with non Sequence of ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.