CHAPTER 1 Synthesis of amides using Lewis acid catalyst: Iodine
... organo-metallic reagents with a carbonyl compound has been widely studied.16 The first example of such a Lewis acid-catalyzed allylation reaction in aqueous medium was again reported by Kobayashi.17 In smooth reaction under the influence of 5 mol% of Sc(OTf)3, tetra allyltin reacted with several ket ...
... organo-metallic reagents with a carbonyl compound has been widely studied.16 The first example of such a Lewis acid-catalyzed allylation reaction in aqueous medium was again reported by Kobayashi.17 In smooth reaction under the influence of 5 mol% of Sc(OTf)3, tetra allyltin reacted with several ket ...
Chemistry 210 - MiraCosta College
... L. The mechanism of acid-catalyzed dehydration of alcohols M. Rearrangements in alcohol dehydration O. Dehydrohalogenation of alkyl halides P. Mechanism of the dehydrohalogenation of alkyl halides: the E2 mechanism Q. Anti-elimination in E2 reactions: stereoelectronic effects R. A different mechanis ...
... L. The mechanism of acid-catalyzed dehydration of alcohols M. Rearrangements in alcohol dehydration O. Dehydrohalogenation of alkyl halides P. Mechanism of the dehydrohalogenation of alkyl halides: the E2 mechanism Q. Anti-elimination in E2 reactions: stereoelectronic effects R. A different mechanis ...
Organic Isomers - Winston Knoll Collegiate
... which the methyl group in alanine has been replaced by a second hydrogen atom do not exist as enantiomers. All other 2-amino acids exist as two enantiomeric forms. Because these molecules are so similar, there is very little difference in their physical and chemical properties. In fact, the only dif ...
... which the methyl group in alanine has been replaced by a second hydrogen atom do not exist as enantiomers. All other 2-amino acids exist as two enantiomeric forms. Because these molecules are so similar, there is very little difference in their physical and chemical properties. In fact, the only dif ...
Physical Science Chapter 7 Chemical Reactions Section 7.1
... A Bunsen burner generates heat and light by the combustion of natural gas. The reaction of hydrogen and oxygen is a combustion reaction. ____________________________________________ You could also classify this reaction as the synthesis of water. _________________________________ ___________________ ...
... A Bunsen burner generates heat and light by the combustion of natural gas. The reaction of hydrogen and oxygen is a combustion reaction. ____________________________________________ You could also classify this reaction as the synthesis of water. _________________________________ ___________________ ...
separation methods
... acetate. Since the solubility of amyl acetate is low, isolation could be performed by a liquid-liquid extraction of erythromycin using water-amyl acetate system. For the extraction, it would be desirable to raise the pH of the aqueous phase above the pKa of erythromycin of 8.8, so that the secondary ...
... acetate. Since the solubility of amyl acetate is low, isolation could be performed by a liquid-liquid extraction of erythromycin using water-amyl acetate system. For the extraction, it would be desirable to raise the pH of the aqueous phase above the pKa of erythromycin of 8.8, so that the secondary ...
100 Problems and Exercises in Organometallic Chemistry Anil J. Elias
... 5. Reduction by sodium amalgam (Na/Hg), of η5-(CH3C5H4)MCl4 compounds (M = Mo, W) were found to give dimeric molecules as products which are at the same time neutral, symmetrical, and tetrachlorinated in nature. Given that the new tungsten complex is a 16 electron species and the molybdenum complex ...
... 5. Reduction by sodium amalgam (Na/Hg), of η5-(CH3C5H4)MCl4 compounds (M = Mo, W) were found to give dimeric molecules as products which are at the same time neutral, symmetrical, and tetrachlorinated in nature. Given that the new tungsten complex is a 16 electron species and the molybdenum complex ...
Air-Stable Trialkylphosphonium Salts
... A. F.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 6989-7000. Shaughnessy, K. H.; Kim, P.; Hartwig, J. F. J. Am. Chem. Soc. 1999, 121, 2123-2132. (d) Stille reaction: Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 1999, 38, 2411-2413. Littke, A. F.; Schwarz, L.; Fu, G. C. Manuscript in preparation. (e) ...
... A. F.; Fu, G. C. J. Am. Chem. Soc. 2001, 123, 6989-7000. Shaughnessy, K. H.; Kim, P.; Hartwig, J. F. J. Am. Chem. Soc. 1999, 121, 2123-2132. (d) Stille reaction: Littke, A. F.; Fu, G. C. Angew. Chem., Int. Ed. 1999, 38, 2411-2413. Littke, A. F.; Schwarz, L.; Fu, G. C. Manuscript in preparation. (e) ...
A Guide to Rate of Reactions
... 4.1 Does the change in concentration of sodium hydroxide / hydrochloric acid affect the rate of reaction? Or What is the relationship between change in concentration of sodium hydroxide /hydrochloric acid and the rate of reaction? 4.2 The rate of reaction increases as the concentration of sodium hyd ...
... 4.1 Does the change in concentration of sodium hydroxide / hydrochloric acid affect the rate of reaction? Or What is the relationship between change in concentration of sodium hydroxide /hydrochloric acid and the rate of reaction? 4.2 The rate of reaction increases as the concentration of sodium hyd ...
NUCLEOPHILIC SUBSTITUTION & ELIMINATION ON Csp 3
... SN2 – putting it all (almost) together: - The substrate: 1o C (or, not so good, 2º C) - The nucleophile: good - The leaving group: low pKa of conjugate acid - The solvent: polar, aprotic (next slides) The reaction flow & the transition state: ...
... SN2 – putting it all (almost) together: - The substrate: 1o C (or, not so good, 2º C) - The nucleophile: good - The leaving group: low pKa of conjugate acid - The solvent: polar, aprotic (next slides) The reaction flow & the transition state: ...
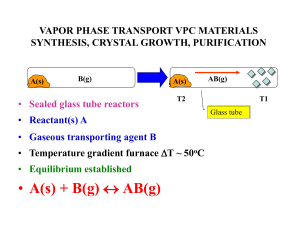
vapor phase transport vpc materials synthesis, crystal growth
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
Chapter 7 Lecture
... show what is happening, spectator ions can be omitted. • Equations such as this one, which show only the species that actually participate in the reaction, are called net ionic equations. Ag+(aq) + Cl− (aq) AgCl(s) ...
... show what is happening, spectator ions can be omitted. • Equations such as this one, which show only the species that actually participate in the reaction, are called net ionic equations. Ag+(aq) + Cl− (aq) AgCl(s) ...
Hydrolases as Catalysts for Green Chemistry and
... The use of enzymes in industrial applications has been recognised for providing clean processes with minimal impact on the environment. This thesis presents studies on engineering of enzymes and enzymebased processes in the light of green chemistry and environmental sustainability, and focuses on th ...
... The use of enzymes in industrial applications has been recognised for providing clean processes with minimal impact on the environment. This thesis presents studies on engineering of enzymes and enzymebased processes in the light of green chemistry and environmental sustainability, and focuses on th ...
Chemistry - Swami Ramanand Teerth Marathwada University
... Concept of entropy: Introduction, Definition, Mathematical Expression, Unit. Entropy as a state function. Entropy changes for reversible and irreversible processes in isolated systems. Entropy change in Physical transformations: (i) Fusion of a solid. (ii) Vaporization of a liquid. (iii) Transition ...
... Concept of entropy: Introduction, Definition, Mathematical Expression, Unit. Entropy as a state function. Entropy changes for reversible and irreversible processes in isolated systems. Entropy change in Physical transformations: (i) Fusion of a solid. (ii) Vaporization of a liquid. (iii) Transition ...
Lecture1
... that would be hard to make otherwise. This is because, compared to "standard organic chemistry", metals display new and unusual reaction types. ...
... that would be hard to make otherwise. This is because, compared to "standard organic chemistry", metals display new and unusual reaction types. ...
Microwave-Assisted Esterification of N -Acetyl-L-Phenylalanine Using Modified Mukaiyama s Reagents: A New Approach Involving Ionic Liquids
... min at 80 °C; 2 [2-ClMePy]I = 2-chloro-1-methylpyridinium iodide; 3 yield was based on the isolated ester; 4 TBA = tributylamine; 5 TEA = triethylamine; 6 MIM = 1methylimidazole; 7 DCM = dichloromethane (extra care should be practiced when performing microwave experiments in DCM!). Many organic solv ...
... min at 80 °C; 2 [2-ClMePy]I = 2-chloro-1-methylpyridinium iodide; 3 yield was based on the isolated ester; 4 TBA = tributylamine; 5 TEA = triethylamine; 6 MIM = 1methylimidazole; 7 DCM = dichloromethane (extra care should be practiced when performing microwave experiments in DCM!). Many organic solv ...
Questions
... State how propanone can be distinguished from propanal using infra-red spectra. You are not expected to give actual absorption values, but you should indicate the bonds in the molecules which would give rise to the distinguishing absorptions. ...
... State how propanone can be distinguished from propanal using infra-red spectra. You are not expected to give actual absorption values, but you should indicate the bonds in the molecules which would give rise to the distinguishing absorptions. ...
Chem 150 Unit 9 - Biological Molecules II
... Carbohydrates Carbohydrates play many important roles in biological systems. They represent the major form of chemical energy for both plants and animals. In plants they represent the end product of photosynthesis, and therefore connect all living systems to the sun’s sustaining light energy. Our di ...
... Carbohydrates Carbohydrates play many important roles in biological systems. They represent the major form of chemical energy for both plants and animals. In plants they represent the end product of photosynthesis, and therefore connect all living systems to the sun’s sustaining light energy. Our di ...
Chapter 11: Alcohols and Ethers
... • Alcohol Groups do not “Survive” Many Organic Reactions • Alkylation (Ether Formation) Protects OH’s During Synthesis • Can Remove the Protecting Group w/ Dilute Aqueous Acid • Generally Dissolve Alcohol in Acid, THEN add Isobutylene • Addition in this Manner Minimizes Isobutylene Dimerization • Le ...
... • Alcohol Groups do not “Survive” Many Organic Reactions • Alkylation (Ether Formation) Protects OH’s During Synthesis • Can Remove the Protecting Group w/ Dilute Aqueous Acid • Generally Dissolve Alcohol in Acid, THEN add Isobutylene • Addition in this Manner Minimizes Isobutylene Dimerization • Le ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.