Syllabus - Chemistry
... Absorption; mechanism of absorption of light Transition moment integral, Einstein's treatment, molar integrated absorption intensity, natural radiative lifetime & the calculation of life times. Excitation; d-d transition, charge transfer & intraligand transitions and selection rules. Excited states; ...
... Absorption; mechanism of absorption of light Transition moment integral, Einstein's treatment, molar integrated absorption intensity, natural radiative lifetime & the calculation of life times. Excitation; d-d transition, charge transfer & intraligand transitions and selection rules. Excited states; ...
ALDOL CONDENSATION
... Like the aldol addition, the Michael reaction may proceed via an enol, silyl enol ether in the Mukaiyama‐Michael addition, or more usually, enolate nucleophile. In the latter case, the stabilized carbonyl compound is deprotonated with a strong base (hard enolization) or with a Lewis acid and a ...
... Like the aldol addition, the Michael reaction may proceed via an enol, silyl enol ether in the Mukaiyama‐Michael addition, or more usually, enolate nucleophile. In the latter case, the stabilized carbonyl compound is deprotonated with a strong base (hard enolization) or with a Lewis acid and a ...
Fundamentals of Organic Chemistry
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS 1 ORGANIC
... 13. When HX adds to an alkyne or alkene, describe how you know which carbon the H adds to and which carbon the X adds to and why. 14. When X2 adds to an alkene, where do the X’s end up? 15. a.) When 1 eq. X2 adds to an alkyne, where do the X’s end up? b.) When excess X2 adds to an alkyne, where do t ...
... 13. When HX adds to an alkyne or alkene, describe how you know which carbon the H adds to and which carbon the X adds to and why. 14. When X2 adds to an alkene, where do the X’s end up? 15. a.) When 1 eq. X2 adds to an alkyne, where do the X’s end up? b.) When excess X2 adds to an alkyne, where do t ...
Slide 1
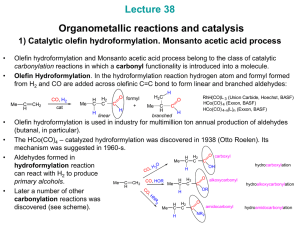
... Olefin hydroformylation and Monsanto acetic acid process belong to the class of catalytic carbonylation reactions in which a carbonyl functionality is introduced into a molecule. Olefin Hydroformylation. In the hydroformylation reaction hydrogen atom and formyl formed from H2 and CO are added across ...
... Olefin hydroformylation and Monsanto acetic acid process belong to the class of catalytic carbonylation reactions in which a carbonyl functionality is introduced into a molecule. Olefin Hydroformylation. In the hydroformylation reaction hydrogen atom and formyl formed from H2 and CO are added across ...
Novel Brønsted-acidic ionic liquids based on benzothiazolium
... had many advantages over reactions catalyzed by conventional liquid or solid acids. In recent years, parallel to the rapid development of ILs, their applications in esterification have been extensively studied. For example, catalytic amounts of Brønsted-acidic ionic liquids promoted esterification, ...
... had many advantages over reactions catalyzed by conventional liquid or solid acids. In recent years, parallel to the rapid development of ILs, their applications in esterification have been extensively studied. For example, catalytic amounts of Brønsted-acidic ionic liquids promoted esterification, ...
Hydrogenation of fatty acid methyl ester to fatty alcohol
... multiphase processes are necessarily operated at high pressures and high hydrogen-to-ester mole ration owing to low solubility of hydrogen in liquid phase. Using the supercritical fluid, a substantially homogeneous supercritical phase could be created, whereby hydrogen could completely access to the ...
... multiphase processes are necessarily operated at high pressures and high hydrogen-to-ester mole ration owing to low solubility of hydrogen in liquid phase. Using the supercritical fluid, a substantially homogeneous supercritical phase could be created, whereby hydrogen could completely access to the ...
PREPARATION OF ORGANOLITHIUM COMPOUNDS - GCG-42
... Since oxygen is more electronegative than carbon, the carbonyl group is electron-deficient at carbon, that is, it is an electrophile. The organometallic compound (R-Li) behaves as a nucleophile with an unshared pair on the carbon. ...
... Since oxygen is more electronegative than carbon, the carbonyl group is electron-deficient at carbon, that is, it is an electrophile. The organometallic compound (R-Li) behaves as a nucleophile with an unshared pair on the carbon. ...
Supported Homogeneous Catalysts
... with polymer ligands, viz. hydrogenation, hydrosilylation, hydroformylation, acetoxylation, polymerisation, and oligomerisation. The published reactions are collected together in the table on pages 70 and 71 with the complex used in the preparation of the supported catalyst, the polymer used, and th ...
... with polymer ligands, viz. hydrogenation, hydrosilylation, hydroformylation, acetoxylation, polymerisation, and oligomerisation. The published reactions are collected together in the table on pages 70 and 71 with the complex used in the preparation of the supported catalyst, the polymer used, and th ...
Document
... • Methanol (CH3OH) • Often Called Wood Alcohol (Distilled From Wood) • Prepared Now via Catalytic Hydrogenation Reactions • Ethanol (CH3CH2OH) • Made Through Fermentation of Sugars, in Alcoholic Drinks • Common Solvent in Organic Labs (Absolute Ethanol) • Ethylene Glycol (HOCH2CH2OH) ...
... • Methanol (CH3OH) • Often Called Wood Alcohol (Distilled From Wood) • Prepared Now via Catalytic Hydrogenation Reactions • Ethanol (CH3CH2OH) • Made Through Fermentation of Sugars, in Alcoholic Drinks • Common Solvent in Organic Labs (Absolute Ethanol) • Ethylene Glycol (HOCH2CH2OH) ...
ch12 by dina
... The starting material may be a ketone or an ester There are two routes that start with ketones (one is shown) ...
... The starting material may be a ketone or an ester There are two routes that start with ketones (one is shown) ...
contents 2002 MAY
... effect of pH and time on the metal removal have also been studied. The metal removal properties are also tested under competitive conditions and found to depend strongly on pH. The polymers possess appreciable selectivity for Pb(II) and Hg(II) over Cd(II) and Cr(VI). The amount of metal uptake by th ...
... effect of pH and time on the metal removal have also been studied. The metal removal properties are also tested under competitive conditions and found to depend strongly on pH. The polymers possess appreciable selectivity for Pb(II) and Hg(II) over Cd(II) and Cr(VI). The amount of metal uptake by th ...
Fundamentals of Organic Chemistry
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
Fundamentals of Organic Chemistry
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
... g. distinguish between ring-activating and ring-deactivating substituents as well as the different directing groups in aromatic disubstitution reactions. ...
Ch. 3 Sections 3.9-3.10 Notes
... number of moles of product that could form? Note the coefficients tell us that 1 mol of N2 consumes 3 mol of H2. 1 mol N2 ↔ 3 mol H2 But 5 mol of H2 was used, not 3, so there will be 2 mol of H2 left over. Once the 1 mol of N2 taken is consumed, no additional NH3 can form. Therefore, the reactant th ...
... number of moles of product that could form? Note the coefficients tell us that 1 mol of N2 consumes 3 mol of H2. 1 mol N2 ↔ 3 mol H2 But 5 mol of H2 was used, not 3, so there will be 2 mol of H2 left over. Once the 1 mol of N2 taken is consumed, no additional NH3 can form. Therefore, the reactant th ...
Carbonyl Compounds_ Properties and Reactions
... Optical activity: The mechanism depends on both the concentration of the nucleophile and the concentration of the carbonyl (it is BIMOLECULAR). The product that results contains a chiral centre (carbonyl carbon) and so will show optical activity. N.B. See nucleophilic substitution reactions of halog ...
... Optical activity: The mechanism depends on both the concentration of the nucleophile and the concentration of the carbonyl (it is BIMOLECULAR). The product that results contains a chiral centre (carbonyl carbon) and so will show optical activity. N.B. See nucleophilic substitution reactions of halog ...
CN>Chapter 22CT>Carbonyl Alpha
... Acetoacetic and malonic esters are easily converted into the corresponding enolate anions by reaction with sodium ethoxide in ethanol. The enolates are good nucleophiles that react rapidly with alkyl halides to give an a-substituted derivatives. The product has an acidic αhydrogen, allowing the alky ...
... Acetoacetic and malonic esters are easily converted into the corresponding enolate anions by reaction with sodium ethoxide in ethanol. The enolates are good nucleophiles that react rapidly with alkyl halides to give an a-substituted derivatives. The product has an acidic αhydrogen, allowing the alky ...
Document
... iodides are several times more reactive than alkyl bromides and from 50 to 100 times more reactive than alkyl chlorides. Fluorine has the strongest bond to carbon, and fluoride is the poorest leaving group. Alkyl fluorides are rarely used as substrates in nucleophilic substitution because they are s ...
... iodides are several times more reactive than alkyl bromides and from 50 to 100 times more reactive than alkyl chlorides. Fluorine has the strongest bond to carbon, and fluoride is the poorest leaving group. Alkyl fluorides are rarely used as substrates in nucleophilic substitution because they are s ...
Enantioselective synthesis

Enantioselective synthesis, also called chiral synthesis or asymmetric synthesis, is defined by IUPAC as: a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereoisomeric) products in unequal amounts.Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer.Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity.