Metal Complexes
... – # ligands = 4 (two en’s, two Cl-’s) – C.N. = 6 (not 4!) because each en ligand makes two coordinate covalent bonds to the Co3+ using two different N atoms per ligand TM I-Intro to Complexes 15 ...
... – # ligands = 4 (two en’s, two Cl-’s) – C.N. = 6 (not 4!) because each en ligand makes two coordinate covalent bonds to the Co3+ using two different N atoms per ligand TM I-Intro to Complexes 15 ...
MS PowerPoint
... Reductive elimination is simply the reverse reaction of oxidative addition. The formal valence state of the metal is reduced by two. And the total electron count of the complex is reduced by two. In a catalytic cycle, the two reactions always occur pair-wise. Reductive eliminations can be promoted b ...
... Reductive elimination is simply the reverse reaction of oxidative addition. The formal valence state of the metal is reduced by two. And the total electron count of the complex is reduced by two. In a catalytic cycle, the two reactions always occur pair-wise. Reductive eliminations can be promoted b ...
1 Carbonyl Condensation Reactions (Conjugate Addition) If we look
... We have seen that “hard” nucleophiles, such as present in RMgBr and RLi reagents, add to the carbonyl. A way to force only conjugate addition with an organometallic reagent is to form the copper salt - in this case, a dialkylcuprate. We’ve already seen these are used to prepare ketones from acid chl ...
... We have seen that “hard” nucleophiles, such as present in RMgBr and RLi reagents, add to the carbonyl. A way to force only conjugate addition with an organometallic reagent is to form the copper salt - in this case, a dialkylcuprate. We’ve already seen these are used to prepare ketones from acid chl ...
18 Valence Electron Rule
... by the metal bound ligands. The counting of the 18 valence electrons in transition metal complexes may be obtained by following either of the two methods of electron counting, (i). the ionic method and (ii). the neutral method. Please note that a metal-metal bond contributes one electron to the to ...
... by the metal bound ligands. The counting of the 18 valence electrons in transition metal complexes may be obtained by following either of the two methods of electron counting, (i). the ionic method and (ii). the neutral method. Please note that a metal-metal bond contributes one electron to the to ...
Predicting synthesis and decomposition reactions
... Not redox means the middle element in the salt will have the same oxidation number at the nonmetal reactant ...
... Not redox means the middle element in the salt will have the same oxidation number at the nonmetal reactant ...
$doc.title
... 1. The octahedral molecular orbital (MO) diagram provides the starting point for the construction of the electronic structure of several metal complexes. But not all complexes are conveniently referenced to octahedral geometry. Other important geometries include tetrahedral, square planar, trigonal ...
... 1. The octahedral molecular orbital (MO) diagram provides the starting point for the construction of the electronic structure of several metal complexes. But not all complexes are conveniently referenced to octahedral geometry. Other important geometries include tetrahedral, square planar, trigonal ...
Transition metal ions
... • So a substance that appears yellow may do so because it absorbs most strongly in the blue part of the spectrum and scatters most strongly in the red and green parts of the spectrum. • It is often the case that a pigment scatters light most efficiently in one region of the spectrum whilst having it ...
... • So a substance that appears yellow may do so because it absorbs most strongly in the blue part of the spectrum and scatters most strongly in the red and green parts of the spectrum. • It is often the case that a pigment scatters light most efficiently in one region of the spectrum whilst having it ...
SYSTEMATIC NOMENCLATURE OF COORDINATION COMPOUNDS
... either one of the O atoms. (known as nitro isomer if linked through nitrogen and nitrito isomer if linked through oxygen) ...
... either one of the O atoms. (known as nitro isomer if linked through nitrogen and nitrito isomer if linked through oxygen) ...
Coordination Chemistry I: Structures and Isomers
... that make up the secondary coordination sphere ...
... that make up the secondary coordination sphere ...
Module 9 : Complexes of π −bound ligands Lecture 2 : Metal allyl
... Figure 1. Metal−allyl interaction. Metal−allyl interaction Of particular interest are the molecular orbitals namely Ψ 1 , Ψ 2 and Ψ 3 of the allyl ligand that interact with the metal in a metal allyl complex. The energy of these molecular orbitals increase with the increase in the number of nodes. ...
... Figure 1. Metal−allyl interaction. Metal−allyl interaction Of particular interest are the molecular orbitals namely Ψ 1 , Ψ 2 and Ψ 3 of the allyl ligand that interact with the metal in a metal allyl complex. The energy of these molecular orbitals increase with the increase in the number of nodes. ...
Lecture notes for chapter 7
... the C=C bond. In the Zr complex, however, we see an interesting reversal where the single bond across the back of the butadiene shortens quite a bit. What is happening here is that the Zr is in a very low oxidation state (+2, but it really wants to be +4) and is, therefore, extremely electron-rich. ...
... the C=C bond. In the Zr complex, however, we see an interesting reversal where the single bond across the back of the butadiene shortens quite a bit. What is happening here is that the Zr is in a very low oxidation state (+2, but it really wants to be +4) and is, therefore, extremely electron-rich. ...
CBSE Class 12 Chemistry notes and questions for
... iii The name of the anionic ligands end in –o, those of neutral and cationic ligands are the same except aqua for H2O, ammine for NH3, carbonyl for CO and nitrosyl for NO. these are placed within enclosing marks . iv When the prefixes mono, di, tri, etc., are used to indicate the number of the indiv ...
... iii The name of the anionic ligands end in –o, those of neutral and cationic ligands are the same except aqua for H2O, ammine for NH3, carbonyl for CO and nitrosyl for NO. these are placed within enclosing marks . iv When the prefixes mono, di, tri, etc., are used to indicate the number of the indiv ...
e-nomenclature-of-coordination-compounds-take-home-2
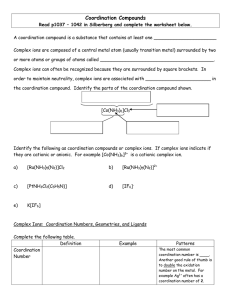
... A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of atoms called ________________________________________. Complex ions can often be recogniz ...
... A coordination compound is a substance that contains at least one ______________________ Complex ions are composed of a central metal atom (usually transition metal) surrounded by two or more atoms or groups of atoms called ________________________________________. Complex ions can often be recogniz ...
Solid phase reactions II
... lack of methods for acide chloride formation mainly two procedures Steglich: DIC/DMAP Yamaguchi: TCBC (2,4,6-trichlorobenzoylchloride) ...
... lack of methods for acide chloride formation mainly two procedures Steglich: DIC/DMAP Yamaguchi: TCBC (2,4,6-trichlorobenzoylchloride) ...
lect7
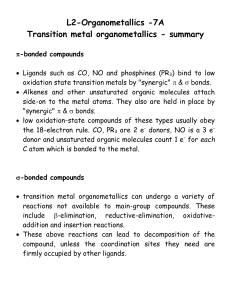
... Ligands such as CO, NO and phosphines (PR3) bind to low oxidation state transition metals by "synergic" & bonds. Alkenes and other unsaturated organic molecules attach side-on to the metal atoms. They also are held in place by "synergic" & bonds. low oxidation-state compounds of these ...
... Ligands such as CO, NO and phosphines (PR3) bind to low oxidation state transition metals by "synergic" & bonds. Alkenes and other unsaturated organic molecules attach side-on to the metal atoms. They also are held in place by "synergic" & bonds. low oxidation-state compounds of these ...
Concepts in Transition Metal Chemistry – Questions
... O2 + 4H+ + 4e = 2H2O also defines the strength of oxygen (in contact with aqueous hydrogen ions) as an oxidising agent. How many of the ions Cr2+(aq), Mn2+(aq), Fe2+(aq) and Co2+(aq) are thermodynamically unstable to oxidation by oxygen in acid solution? ...
... O2 + 4H+ + 4e = 2H2O also defines the strength of oxygen (in contact with aqueous hydrogen ions) as an oxidising agent. How many of the ions Cr2+(aq), Mn2+(aq), Fe2+(aq) and Co2+(aq) are thermodynamically unstable to oxidation by oxygen in acid solution? ...
Cu II complex - IONiC / VIPEr
... relationship that provides the selectivity for Cu(I). It also provides insight into potential mechanisms of heavy metal toxicity in this and other biochemical pathways. ...
... relationship that provides the selectivity for Cu(I). It also provides insight into potential mechanisms of heavy metal toxicity in this and other biochemical pathways. ...
Properties of the transition metals
... transitions can occur between them. These transitions require energy provided by light from the visible portion of the electromagnetic spectrum (this has the correct amount of energy). Hence the transition metal ions absorb part of the visible spectrum and we see only those parts of the spectrum whi ...
... transitions can occur between them. These transitions require energy provided by light from the visible portion of the electromagnetic spectrum (this has the correct amount of energy). Hence the transition metal ions absorb part of the visible spectrum and we see only those parts of the spectrum whi ...
Document
... Reaction with a hydride – reduction to alcohol • Lithium aluminium hydride (LiAlH4) and sodium borohydride (NaBH4) reduce aldehydes and ketones to alcohols. • Both species can be simplified as H- which is the nucleophile that adds to the carbonyl. • Attack at the electron poor C of the C=O gives an ...
... Reaction with a hydride – reduction to alcohol • Lithium aluminium hydride (LiAlH4) and sodium borohydride (NaBH4) reduce aldehydes and ketones to alcohols. • Both species can be simplified as H- which is the nucleophile that adds to the carbonyl. • Attack at the electron poor C of the C=O gives an ...
MSWord
... RN=C(PR2)(NHR), an important class of heteroatom-containing compounds which may be used as building blocks for organic synthesis and as unique ligands for various metal complexes. However, such catalytic process has been hardly explored. We report here a novel catalytic synthesis of phosphaguanidi ...
... RN=C(PR2)(NHR), an important class of heteroatom-containing compounds which may be used as building blocks for organic synthesis and as unique ligands for various metal complexes. However, such catalytic process has been hardly explored. We report here a novel catalytic synthesis of phosphaguanidi ...
Tertiary Phosphine Complexes of the Platinum Metals
... as a nucleophile; molecules such as H, or 0, ...
... as a nucleophile; molecules such as H, or 0, ...
Stability of Transition Metal Complexes
... Hard donors: electronegative, not easily polarized F, O, N, Cl, OH-, π-donors Soft (Class b) metals: low Q/r ratio, not very polarizing zero oxidation state or late d block, p block metals prefer Soft donors: medium electronegativity, easily polarized, π-acceptors I, S, P, H-, CO, alkenes Intermedia ...
... Hard donors: electronegative, not easily polarized F, O, N, Cl, OH-, π-donors Soft (Class b) metals: low Q/r ratio, not very polarizing zero oxidation state or late d block, p block metals prefer Soft donors: medium electronegativity, easily polarized, π-acceptors I, S, P, H-, CO, alkenes Intermedia ...
Metal carbonyl

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel carbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometalic complexes.Metal carbonyls are toxic by skin contact, inhalation or ingestion, in part because of their ability to carbonylate hemoglobin to give carboxyhemoglobin, which prevents the binding of O2.