* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cu II complex - IONiC / VIPEr
Survey
Document related concepts
Transcript
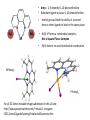
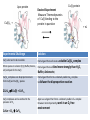
Ligands that Favor/Force the Formation of Tetrahedral Complexes with an Application in Bioinorganic Chemistry Marion E. Cass, Carleton College Michael J. Stevenson, Dartmouth College Molly L. Croteau, Dartmouth College Created by Michael J. Stevenson, Dartmouth College ([email protected]) Molly L. Croteau, Dartmouth College ([email protected]) and Marion E. Cass, Carleton College ([email protected]), and posted on VIPEr on April 18, 2016. Copyright Michael J. Stevenson, Molly L. Croteau, and Marion E. Cass. This work is licensed under the Creative Commons Attribution Non-commercial Share Alike License. To view a copy of this license visit http://creativecommons.org/about/license/. • dmp = 2,9-dimethyl-1,10-phenanthroline • Bidentate ligand w planar 1,10-phenanthroline • M methyl groups block the ability of a second dmp or other ligands to bind in the same plane • Ni(II) d8 forms a tetrahedral complex: Not a Square Planar Complex • Pt(II) distorts to avoid tetrahedral coordination NiIIdmpI2 PtIIdmpI2 For all 3D JSmol movable images addressed in this LO see: http://www.people.carleton.edu/~mcass/1-InorganicC351/jsmol/LigandsFavoringTetrahedralGeometry.htm Several other ligands that force tetrahedral geometry are known See: “A paramagnetic tetrahedral Pd(II) complex” Housecroft and Sharpe, Inorganic Chemistry 3rd Ed, p. 671 CCDC Reference: DICSIR J.S.L.Yeo, J.J.Vittal, T.S.A.Hor, Chem Commun. 1999, p. 1477 For a 3D JSmol movable image see: http://www.people.carleton.edu/~mcass/1-InorganicC351/jsmol/LigandsFavoringTetrahedralGeometry.htm Dichloro-(1,1'-bis(oxodiphenylphosphoranyl)ferrocenyl)-palladium(II) A Bio-inorganic Application Dean Wilcox and his students at Dartmouth College carry out careful thermodynamics measurements to examine metal binding in metalloproteins Molly Croteau examines the binding of metal ions to Azurin (a blue copper electron transport protein). In azurin the copper metal ion shuttles between Cu(I) and Cu(II) in order to provide electrons to cytochrome c oxidase in bacterial cells. Binding of copper in both oxidation states to the apo-azurin is essential for understanding how this protein tunes its reduction potential to participate with cytochrome c oxidase in vivo. Michael Stevenson studies the binding of various metal ions to the copper metallochaperone protein HAH1. The reducing environment of the cell makes the predominant copper oxidation state to be Cu(I). HAH1 is finely tuned to bind Cu(I) and transport it through the cytosol for delivery to the trans Golgi network. Competition by other metal ions provides insight into ferreting out the structure/thermodynamic relationship that provides the selectivity for Cu(I). It also provides insight into potential mechanisms of heavy metal toxicity in this and other biochemical pathways. In both instances, it is crucial to know the oxidation state of the metal being delivered during a carefully controlled experiment. For Cu(I), this is not an easy experimental task. Apo-protein Cu(I)Ln + Desired Experiment Measure Thermodynamics of Cu(I) binding to the protein in question Cu(I)-protein Experimental Challenge Solution Cu(I) salts tend to be insoluble Find a ligand that will create a soluble Cu(I)Ln complex Other species in solution (H2O, Buffer, Anions, etc) compete for the Cu(I) Find a ligand that will bind more strongly than H2O, Cu(I)Ln complexes can disproportionate to form Cu(0) and Cu(II)Ln species Find a ligand that forms a relatively stable CuILn complex to disfavor the disproportion reaction Buffer, Anions etc) 2CuILn ⇌ Cu(0) + CuIILn Cu(I) complexes can be oxidized in the presence of O2 CuILn + O2 CuIILn Again use a ligand that forms a relatively stable CuILn complex However most importantly, work in an O2 free environment + nL Ligand 1: Me6Trien CuIMe6Trien will not disproportionate 2CuIL + Cu(0) + CuIIL2+ And in fact: the reverse comproportion reaction is the preferred preparation method CuII + Cu(0) + excess Me6Trien in O2 If O2 free CuIL is the predominant Species in solution For 3D JSmol movable images see: http://www.people.carleton.edu/~mcass/1-Inorganic-C351/jsmol/LigandsFavoringTetrahedralGeometry.htm 2 CuIL+ The Cu(I) will oxidize to Cu(II) and will pick up an additional L: anion or solvent Ligand 2: BCA [CuIBCA2]3-will not disproportionate 2[CuIBCA2]3- Cu(0) + [CuIIBCA2]2- CuI complex CuII complex in O2 The CuII complex is believed to have a similar 3D structure to the Cu(I) complex. The crystal structure of the complex with one BCA analog and two Cl- Ligands is shown below If O2 free [CuIBCA2]3- is the predominant species in soln For 3D JSmol movable images see: http://www.people.carleton.edu/~mcass/1-Inorganic-C351/jsmol/LigandsFavoringTetrahedralGeometry.htm Ligand 3: BCS [CuIBCS2]3-will not disproportionate 2[CuIBCS2]3- Cu(0) + [CuIIBCS2]2- CuI complex CuII complex in O2 If O2 free [CuIBCS2]3- is the predominant species in soln For 3D JSmol movable images see: http://www.people.carleton.edu/~mcass/1-Inorganic-C351/jsmol/LigandsFavoringTetrahedralGeometry.htm In Summary: All Three ligands form relatively stable CuI complexes in the absence of O2 Me6Trien BCA BCS 1. What are the similarities in the 3 complexes? 2. Suggest why the CuI complexes are “relatively stable” (meaning relative to its CuII complex under the controlled experimental conditions). 3. Suggest why they are soluble in H2O. 4. It turns out for the reaction: CuI + n L CuILn Kf(CuIMe6Trien) < Kf(CuIBCA2) < Kf(CuIBCS2) Suggest why it is useful to have 3 ligands with 3 differing formation constants. If you had to rationalize the relative order of the formation constants what would you suggest? 5. When oxidized, the CuII complex of Me6Trien has a different geometry than the CuI complex. Suggest why this occurs with Me6Trien but the distortion is less pronounced with BCA or BCS? References and Resources • D.K. Johnson, M. J. Stevenson, Z.A. Almadidy, S.E. Jenkins, D. E. Wilcox, N. E. Grossoeheme; “Stabilization of Cu(I) for binding and calorimetric measurements in aqueous solution” Dalton Transactions, 2015, Vol 44, Issue 37, p. 16494-16505 (DOI 10.1039/c5dt02689) • A very neat application of the shuttling of the CuI/CuII complexes with Me6Trien and other ligands is for use in radical polymerization reactions: See G. Kickelbick, T. Pintauerb and K. Matyjaszewski; “Structural comparison of CuII complexes in atom transfer radical polymerization” New J. Chem, 2002, 26, p. 462-468 (DOI 10.1039/b105454f)