
Acid derivatives
... and bond-making occuring simultaneously (the SN2 mechanism) were observed. On the other hand, for most cases of electrophilic aromatic substitution bond-making preceded bond-breaking. As illustrated in the following diagram, acylation reactions generally take place by an addition-elimination process ...
... and bond-making occuring simultaneously (the SN2 mechanism) were observed. On the other hand, for most cases of electrophilic aromatic substitution bond-making preceded bond-breaking. As illustrated in the following diagram, acylation reactions generally take place by an addition-elimination process ...
(Z)-Tamoxifen and Tetrasubstituted Alkenes and Dienes via a Regio
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
Asymmetric Synthesis: Substrate and Auxiliary Control
... ▪ New synthetic methods mean that some other chiral molecules are now extremely cheap, and are now considered as a new chiral pool. ...
... ▪ New synthetic methods mean that some other chiral molecules are now extremely cheap, and are now considered as a new chiral pool. ...
aldehydes and ketones
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
... Lower members of aldehydes and ketones ( upto C4) are soluble in water due to H-bonding between polar carbonyl group and water. However, solubility decreases with increase in molecular weight Aromatic aldehydes and ketones are much less soluble than corresponding aliphatic aldehydes and ketone ...
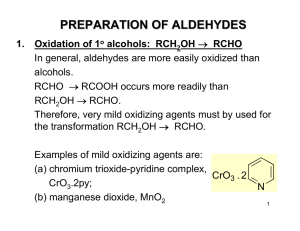
PREPARATION OF ALDEHYDES
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
Hydroxyl-Directed Stereoselective Diboration of Alkenes
... competition 1) than tetradecene (12% conversion). This experiment establishes a lower limit for the reaction rate ratio of >∼4. In line with observed selectivity, a similar experiment showed that a bishomoallylic alcohol reacts at a rate comparable to the homoallylic alcohol (competition 2), whereas ...
... competition 1) than tetradecene (12% conversion). This experiment establishes a lower limit for the reaction rate ratio of >∼4. In line with observed selectivity, a similar experiment showed that a bishomoallylic alcohol reacts at a rate comparable to the homoallylic alcohol (competition 2), whereas ...
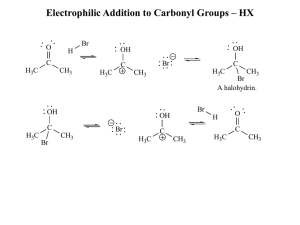
Electrophilic Addition to Carbonyl Groups – HX
... and Aldol Reactions • This reaction is known as the aldol reaction. It takes an aldehyde and converts it into an alcohol (while extending the carbon chain). • If an aldol reaction is worked up under acidic conditions, an E2 reaction will follow, giving a double bond conjugated to the carbonyl group: ...
... and Aldol Reactions • This reaction is known as the aldol reaction. It takes an aldehyde and converts it into an alcohol (while extending the carbon chain). • If an aldol reaction is worked up under acidic conditions, an E2 reaction will follow, giving a double bond conjugated to the carbonyl group: ...
CBSEGuess.com
... --------------------------------------------------------------------------------------------------------------------------------------1. Why sulphuric acid is not used during the reaction of alcohols with KI? 2. Explain swarts reaction. 3. How o- and p- niterophenols are separated? 4. Conert (a) eth ...
... --------------------------------------------------------------------------------------------------------------------------------------1. Why sulphuric acid is not used during the reaction of alcohols with KI? 2. Explain swarts reaction. 3. How o- and p- niterophenols are separated? 4. Conert (a) eth ...
No Slide Title
... Concentrated acids are hydrophilic and will remove water from other molecules in order to dilute themselves. ...
... Concentrated acids are hydrophilic and will remove water from other molecules in order to dilute themselves. ...
Some uses of mischmetall in organic synthesis
... reduction-transmetallation process with Ln, provides another lanthanide alkoxide, with the regeneration of SmI2 (or SmX2). In the catalytic scheme II, the initially formed RSmIX reacts with Ln to give another organometallic compound (LnR3; Ln ≠ Sm) through a reduction-transmetallation process, with ...
... reduction-transmetallation process with Ln, provides another lanthanide alkoxide, with the regeneration of SmI2 (or SmX2). In the catalytic scheme II, the initially formed RSmIX reacts with Ln to give another organometallic compound (LnR3; Ln ≠ Sm) through a reduction-transmetallation process, with ...
Ethers and Epoxides
... • Three membered ring ether is called an oxirane (root “ir” from “tri” for 3-membered; prefix “ox” for oxygen; “ane” for saturated) • Also called epoxides • Ethylene oxide (oxirane; 1,2-epoxyethane) is industrially important as an intermediate • Prepared by reaction of ethylene with oxygen at 300 °C ...
... • Three membered ring ether is called an oxirane (root “ir” from “tri” for 3-membered; prefix “ox” for oxygen; “ane” for saturated) • Also called epoxides • Ethylene oxide (oxirane; 1,2-epoxyethane) is industrially important as an intermediate • Prepared by reaction of ethylene with oxygen at 300 °C ...
Organocatalysed asymmetric Mannich reactions
... 3-amino tetroses 26 was reported by Córdova and co-workers. 3-Aminotetroses 26 were obtained through a proline-catalysed homo-Mannich reaction of protected glycolaldehydes 28 (Scheme 9).12 The enantioselectivity of this reaction was high (up to .99% ee), but the diastereoselectivity disappointing w ...
... 3-amino tetroses 26 was reported by Córdova and co-workers. 3-Aminotetroses 26 were obtained through a proline-catalysed homo-Mannich reaction of protected glycolaldehydes 28 (Scheme 9).12 The enantioselectivity of this reaction was high (up to .99% ee), but the diastereoselectivity disappointing w ...
Links - American Chemical Society
... The tolerance of aryl and alkyl bromides, alkyl iodides, and aryl fluorides is especially noteworthy, as several of these transformations were previously6c carried out in the presence of PMHS only with difficulty and concomitant hydrodehalogenation. The selective reduction of methyl esters in the pr ...
... The tolerance of aryl and alkyl bromides, alkyl iodides, and aryl fluorides is especially noteworthy, as several of these transformations were previously6c carried out in the presence of PMHS only with difficulty and concomitant hydrodehalogenation. The selective reduction of methyl esters in the pr ...
12-Nucleophilic Reactions
... Thus initially in reaction (when [Br-] is zero), the expression simplifies to the traditional first order kinetics expression, but as reaction proceeds the rate will decrease as [Br-] increases or if extra [Br-] is added to the reaction (called common ion rate depression) ...
... Thus initially in reaction (when [Br-] is zero), the expression simplifies to the traditional first order kinetics expression, but as reaction proceeds the rate will decrease as [Br-] increases or if extra [Br-] is added to the reaction (called common ion rate depression) ...
Chem 314 Preorganic Evaluation
... special features: biomolecular kinetics (Rate = kE2[RX][B-], single step concerted reaction, competing reaction is SN2 favored reactivity: 3oRX > 2o RX > 1oRX (none at CH3X, need Cβ-H), 1oRX will produce mainly SN2 product excet for mostly E2 with the sterically hindered and highly basic potassium t ...
... special features: biomolecular kinetics (Rate = kE2[RX][B-], single step concerted reaction, competing reaction is SN2 favored reactivity: 3oRX > 2o RX > 1oRX (none at CH3X, need Cβ-H), 1oRX will produce mainly SN2 product excet for mostly E2 with the sterically hindered and highly basic potassium t ...
Chapter 8 I. Nucleophilic Substitution
... Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected m ...
... Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected m ...
Alkyl halide
... forming an allylic radical plus HBr • The allylic radical reacts with Br2 to yield the product and a Br. radical which cycles back to carry on the chain • Br2 results from reaction of NBS with the HBr formed in the first ...
... forming an allylic radical plus HBr • The allylic radical reacts with Br2 to yield the product and a Br. radical which cycles back to carry on the chain • Br2 results from reaction of NBS with the HBr formed in the first ...
Carbonyl α-substitution and Condensation Reactions
... is to look at the acidity of the corresponding ammonium ion ...
... is to look at the acidity of the corresponding ammonium ion ...
Guide_to_Life_in_Orgo_Ib
... shoulders - Good preparation is key for doing well. With this in mind, when you look at the course outline over the next few pages, you will see that the semester is split up into separate lessons, and the content we will go through in each lesson; there are also preparatory assignments listed for e ...
... shoulders - Good preparation is key for doing well. With this in mind, when you look at the course outline over the next few pages, you will see that the semester is split up into separate lessons, and the content we will go through in each lesson; there are also preparatory assignments listed for e ...
Forward
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
... As we’ll see later in this chapter and the next, aldehydes and ketones are involved in many of the most used reactions in synthetic organic chemistry. Where do aldehydes and ketones come from? Many occur naturally. In terms of both variety and quantity, aldehydes and ketones rank among the most comm ...
Catalytic Functionalization of Methyl Group on Silicon: Iridium
... organosilicon compounds have been synthesized from methylchlorosilanes and utilized in organic and inorganic synthesis.2In these applications, the conversion of methylchlorosilanes is based on reactions at their Si−Cl bonds, which are efficiently converted into Si−O, Si−N, and Si−C bonds. In contrast, ...
... organosilicon compounds have been synthesized from methylchlorosilanes and utilized in organic and inorganic synthesis.2In these applications, the conversion of methylchlorosilanes is based on reactions at their Si−Cl bonds, which are efficiently converted into Si−O, Si−N, and Si−C bonds. In contrast, ...








![ch13[1].](http://s1.studyres.com/store/data/008194698_1-d188c504eac7b7806e762a2340484910-300x300.png)











