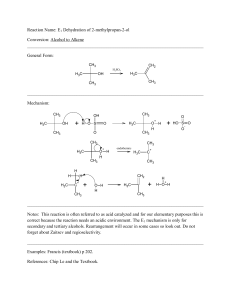
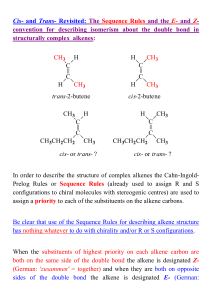
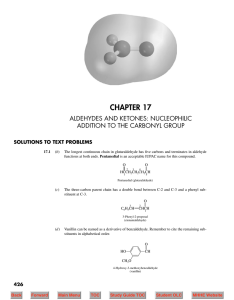
CH 2 - ResearchGate
... It has been developed by H.E. Simmons and R.D. Smith of the DuPont Company. During this synthesis diiodomethane and zinc-copper couple are stirred together with alkene. The diiodomethane and zinc react together to produce carbene like species called as carbenoid which brings stereospecific addition ...
... It has been developed by H.E. Simmons and R.D. Smith of the DuPont Company. During this synthesis diiodomethane and zinc-copper couple are stirred together with alkene. The diiodomethane and zinc react together to produce carbene like species called as carbenoid which brings stereospecific addition ...
Electrophilic Selenium Catalysis with Electrophilic N
... Functionalization of alkenes is a perpetual goal in organic synthesis. One of the attractive routes to elaborate the carbon–carbon double bond of alkenes is through electrophilic selenium reagent-promoted selenofunctionalization. In this context, several electrophilic organoselenium reagents ArSeX ( ...
... Functionalization of alkenes is a perpetual goal in organic synthesis. One of the attractive routes to elaborate the carbon–carbon double bond of alkenes is through electrophilic selenium reagent-promoted selenofunctionalization. In this context, several electrophilic organoselenium reagents ArSeX ( ...
ALDEHYDES AND KETONES I. NUCLEOPHILIC ADDITION TO …
... •Once the substrate (aldehyde or ketone) is bound to the enzyme, the active site of the enzyme is in a position to react with and modify the substrate. •At the end of the reaction, because imines come apart easily (remember the “unfavorable” equilibrium?), the modified substrate can dissociate from ...
... •Once the substrate (aldehyde or ketone) is bound to the enzyme, the active site of the enzyme is in a position to react with and modify the substrate. •At the end of the reaction, because imines come apart easily (remember the “unfavorable” equilibrium?), the modified substrate can dissociate from ...
Module 2 Asymmetric Carbon-Carbon Bond Forming Reactions
... 2.2 Enantioselective Alkene Metathesis Among the alkene metathesis catalysts, Mo and Ru-based complexes have emerged as powerful exhibiting complementary reactivity and functional group tolerance. ...
... 2.2 Enantioselective Alkene Metathesis Among the alkene metathesis catalysts, Mo and Ru-based complexes have emerged as powerful exhibiting complementary reactivity and functional group tolerance. ...
Synthesis of Natural Products and Related Compounds using Enyne
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
Alkenes 3 - ChemWeb (UCC)
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
... This reaction illustrated above is called a 1,2- or -elimination to indicate that the groups being eliminated are located on adjacent atoms in the starting material as compared to a 1,1- or -elimination where both are located on the same carbon atom This, in itself, tells you nothing about the act ...
Organic Chemistry II
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
... Alkyl Halides We did talk about halo-alkanes (called alkyl halides) which are alkanes with a halogen attached. These molecules do, in fact, have polar bonds: C-Br, C-I, C-Cl are all polar bonds. Carbon is slightly positive, the halogen is slightly negative. ...
IOSR Journal of Applied Chemistry (IOSR-JAC)
... Michael addition reaction from suitably substituted alkene (31) using Fe(NO3)3 as catalyst (Scheme 12).60 This method was exclusively used for synthesis of pyrrolidine. An intramolecular ring expansion strategy of epoxide has been utilised using Fe-NHC catalytic condition, where FeCl2 was used as pr ...
... Michael addition reaction from suitably substituted alkene (31) using Fe(NO3)3 as catalyst (Scheme 12).60 This method was exclusively used for synthesis of pyrrolidine. An intramolecular ring expansion strategy of epoxide has been utilised using Fe-NHC catalytic condition, where FeCl2 was used as pr ...
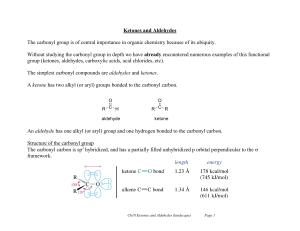
Ketones and Aldehydes
... The base catalyzed addition reactions to carbonyl compounds result from initial attack of a strong nucleophile, whereas the acid catalyzed reactions begin with the protonation of the oxygen, followed by attack of the weaker nucleophile. ...
... The base catalyzed addition reactions to carbonyl compounds result from initial attack of a strong nucleophile, whereas the acid catalyzed reactions begin with the protonation of the oxygen, followed by attack of the weaker nucleophile. ...
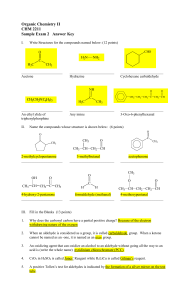
Organic Chemistry II CHM 2211 Sample Exam 2 Answer Key
... Why does the carbonyl carbon have a partial positive charge? Because of the electron withdrawing nature of the oxygen ...
... Why does the carbonyl carbon have a partial positive charge? Because of the electron withdrawing nature of the oxygen ...
Preparation of ethers
... the reactants in a liquid phase. symmetrical or unsymmetrical in nature. Speci?cally The process of this invention may be effected in any speaking the preparation of these ethers is accomplished 65 suitable manner and may comprise either a batch or con tinuous type operation. For example, when a bat ...
... the reactants in a liquid phase. symmetrical or unsymmetrical in nature. Speci?cally The process of this invention may be effected in any speaking the preparation of these ethers is accomplished 65 suitable manner and may comprise either a batch or con tinuous type operation. For example, when a bat ...
Development of New Synthetic Routes to Organoboronates by Catalytic Allylic Substitution and
... To access simple boronates such as allyl (R1=H, R2=H, Scheme 9) and crotyl (R1=Me, R2=H, Scheme 9) species, this method has the advantage of using readily available and inexpensive starting materials. However, these reactive species are known to undergo facile metallotropic rearrangements,109-111 wh ...
... To access simple boronates such as allyl (R1=H, R2=H, Scheme 9) and crotyl (R1=Me, R2=H, Scheme 9) species, this method has the advantage of using readily available and inexpensive starting materials. However, these reactive species are known to undergo facile metallotropic rearrangements,109-111 wh ...
Document
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
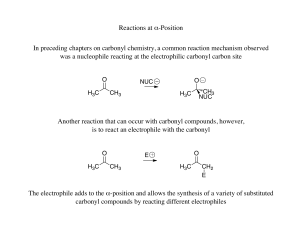
Reactions at α-Position In preceding chapters on carbonyl chemistry
... While reacting two different ketones with alkoxide base is impractical due to the variety of products obtained, the desired product would only be obtained in low yield after a difficult separation, there are methods to react two different carbonyls in an aldol reaction efficiently ...
... While reacting two different ketones with alkoxide base is impractical due to the variety of products obtained, the desired product would only be obtained in low yield after a difficult separation, there are methods to react two different carbonyls in an aldol reaction efficiently ...
Handout VI
... 6.2.1.2 Reduction of Acid Chloride Aldehydes can be prepared by the reduction of acid chloride with hydrogen in xylene using a palladium catalyst suspended on barium sulphate (Rosenmund reduction) (Scheme 4). This process demonstrates the control of chemoselectivity by poisoning of the catalyst. The ...
... 6.2.1.2 Reduction of Acid Chloride Aldehydes can be prepared by the reduction of acid chloride with hydrogen in xylene using a palladium catalyst suspended on barium sulphate (Rosenmund reduction) (Scheme 4). This process demonstrates the control of chemoselectivity by poisoning of the catalyst. The ...
Studies toward the Stereoselective Synthesis of the
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...