aldehydes and ketones
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
Grignard Reaction - OpenBU
... Despite more than a century of organic chemistry, the formation of C–C bonds is still one of the most difficult transformations that we encounter today. Fortunately, the ability of carbon to make bonds not only to C, H, N, O, S, and P (those elements found extensively in the living world), but also ...
... Despite more than a century of organic chemistry, the formation of C–C bonds is still one of the most difficult transformations that we encounter today. Fortunately, the ability of carbon to make bonds not only to C, H, N, O, S, and P (those elements found extensively in the living world), but also ...
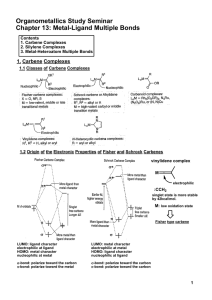
Metal-Ligand Multiple Bonds
... In general, multiple bonds to silicon and other second-row main group elements are less stable than multiple bonds to carbon, oxygen and nitrogen. ...
... In general, multiple bonds to silicon and other second-row main group elements are less stable than multiple bonds to carbon, oxygen and nitrogen. ...
Topic Selection Menu - Pennsylvania State University
... Topic 10: Conjugated Systems Conjugated Systems – Localized and Delocalized Systems – Orbital Diagrams ...
... Topic 10: Conjugated Systems Conjugated Systems – Localized and Delocalized Systems – Orbital Diagrams ...
Topic Selection Menu - Pennsylvania State University
... – Localized and Delocalized Systems – Orbital Diagrams • Bonding, HOMO orbitals • Antibonding LUMO orbitals ...
... – Localized and Delocalized Systems – Orbital Diagrams • Bonding, HOMO orbitals • Antibonding LUMO orbitals ...
Silicon hydrides in organic synthesis
... In our initial experiments we found, in agreement with earlier report^,'^",^ that phenylsilane indeed reacts very sluggishly at room temperature with iodine in chloroform, in benzene or even neat. No significant enhancement of rate was observed either by heating the solution to 60-8OoC or by irradia ...
... In our initial experiments we found, in agreement with earlier report^,'^",^ that phenylsilane indeed reacts very sluggishly at room temperature with iodine in chloroform, in benzene or even neat. No significant enhancement of rate was observed either by heating the solution to 60-8OoC or by irradia ...
Chapter 1
... Rules directly analogous to those for aldehydes • Base name: longest chain with the C=O pent • Replace the –e of alkane name with –one • Indicate position of C=O by number on chain so that C=O has lowest possible number 2 ...
... Rules directly analogous to those for aldehydes • Base name: longest chain with the C=O pent • Replace the –e of alkane name with –one • Indicate position of C=O by number on chain so that C=O has lowest possible number 2 ...
Exam 1 Review Sheet Chapter 15 Chemistry 110b
... Preparation of carboxylic acids. Review the synthetic methods (6 review reactions) and know the scope and mechanisms of reaction for the two new methods we discussed: (i) carbonation of a Grignard reagent and (ii) acid/base hydrolyses of nitriles. [10e, 789-791; 11e, 781-784] ...
... Preparation of carboxylic acids. Review the synthetic methods (6 review reactions) and know the scope and mechanisms of reaction for the two new methods we discussed: (i) carbonation of a Grignard reagent and (ii) acid/base hydrolyses of nitriles. [10e, 789-791; 11e, 781-784] ...
TOPIC 6. NUCLEOPHILIC SUBSTITUTIONS (chapter 6 and parts of
... molecules in one step by substitution of an appropriate material 4. Explore the substitution chemistry of alcohols and ethers ...
... molecules in one step by substitution of an appropriate material 4. Explore the substitution chemistry of alcohols and ethers ...
Reaction of orthoesters with alcohols in the presence of acidic
... known reaction, we were curious to explore this to find out whether such a transformation could be useful as a synthetic methodology and the results are summarized in Table I. Thus the reaction of alcohols and orthoester with various acid catalysts give following products at ambient temperature; uns ...
... known reaction, we were curious to explore this to find out whether such a transformation could be useful as a synthetic methodology and the results are summarized in Table I. Thus the reaction of alcohols and orthoester with various acid catalysts give following products at ambient temperature; uns ...
Chapter 12- Alcohols from Carbonyl Compounds, Redox Reactions
... to being a strong nucleophile, they are also a very strong base • It is not possible to prepare a Grignard from an organic compound that contains an acidic hydrogen • By acidic Hydrogen, we mean any hydrogen that is more acidic than the hydrogens on an alkane or ...
... to being a strong nucleophile, they are also a very strong base • It is not possible to prepare a Grignard from an organic compound that contains an acidic hydrogen • By acidic Hydrogen, we mean any hydrogen that is more acidic than the hydrogens on an alkane or ...
Chapter 1 Organoaluminum Reagents for Selective Organic
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
... Chapter 3. Bulky Aluminum Reagents for Selective Organic Synthesis In chapter 2 we discussed several excellent methods of discriminating various functional groups using bulky aluminum reagents. In this section we focus on the reactions promoted with bulky aluminum reagents which could not be achiev ...
Chapter 18 – Carbonyl Compounds II (Last Chapter we mostly talk
... (It turns out that Schiff bases or Imines C=N very much resembles a C=O bond in reactivity.) - C=N bond similar in nature to C=O bond: ...
... (It turns out that Schiff bases or Imines C=N very much resembles a C=O bond in reactivity.) - C=N bond similar in nature to C=O bond: ...
Chapter 19. Aldehydes and Ketones: Nucleophilic Addition Reactions
... aldehydes Electron-donating resonance effect of aromatic ring makes C=O ...
... aldehydes Electron-donating resonance effect of aromatic ring makes C=O ...
Nuggets of Knowledge for Chapter 14 – Ethers
... Formation and reaction of epoxides is a good way to form trans diols. This is complementary to hydroxylation of alkenes, which gives cis diols. ...
... Formation and reaction of epoxides is a good way to form trans diols. This is complementary to hydroxylation of alkenes, which gives cis diols. ...
proline catalyzed direct asymmetric aldol and mannich reactions
... investigated further by a small group of chemists. Scope of the proline-catalyzed aldol reaction Anti-1,2-diols were easily obtained from hydroxyacetone and various α-substituted ketones as donors. All the aldehyde acceptors but one used were branched at the α position, and no linear aldehyde accept ...
... investigated further by a small group of chemists. Scope of the proline-catalyzed aldol reaction Anti-1,2-diols were easily obtained from hydroxyacetone and various α-substituted ketones as donors. All the aldehyde acceptors but one used were branched at the α position, and no linear aldehyde accept ...