Wed March 3 lecture
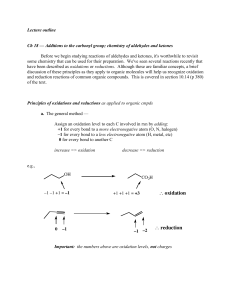
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
... Before we begin studying reactions of aldehydes and ketones, it's worthwhile to revisit some chemistry that can be used for their preparation. We've seen several reactions recently that have been described as oxidations or reductions. Although these are familiar concepts, a brief discussion of these ...
Lab 9 - Academic Computer Center
... addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the three hydride reagents and is easy to use in the lab, because it is soluble in ...
... addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the three hydride reagents and is easy to use in the lab, because it is soluble in ...
Handout 7
... In conclusion, all steps included in the conversion of an aldehyde or ketone to acetal or ketal via hemiacetal or hemiketal as intermediates, are reversible. Performing the reaction in large excess of an anhydrous alcohol and a small amount of an anhydrous acid will strongly favour the formation of ...
... In conclusion, all steps included in the conversion of an aldehyde or ketone to acetal or ketal via hemiacetal or hemiketal as intermediates, are reversible. Performing the reaction in large excess of an anhydrous alcohol and a small amount of an anhydrous acid will strongly favour the formation of ...
Chapter 18 Ketones and Aldehydes 1) Which of the following
... 60) Which series of reactions described below, if any, will result in the formation of 2-methylpentan-3one starting with 1-propanol? A) 1. (CH3)2CHMgBr/ diethyl ether ...
... 60) Which series of reactions described below, if any, will result in the formation of 2-methylpentan-3one starting with 1-propanol? A) 1. (CH3)2CHMgBr/ diethyl ether ...
Reductive etherification of substituted cyclohexanones with
... transfer to yield ethers. Until now two catalysts were always required to achieve this, i.e. a strong acid for the acetalisation and a transition metal for the reduction step.7,8 Various solid catalysts including zeolites have been applied in acid-catalysed acetal fomation9 and the MPV reaction.10,1 ...
... transfer to yield ethers. Until now two catalysts were always required to achieve this, i.e. a strong acid for the acetalisation and a transition metal for the reduction step.7,8 Various solid catalysts including zeolites have been applied in acid-catalysed acetal fomation9 and the MPV reaction.10,1 ...
The Aldol Condensation Preparation of 4
... Preparation of 4-Methoxybenzalacetone In this experiment 4-Methoxybenzalacetone, obtained through an aldol condensation of 4-Methoxybenzaldehyde and Acetone, will be synthesized in a one pot reaction. ...
... Preparation of 4-Methoxybenzalacetone In this experiment 4-Methoxybenzalacetone, obtained through an aldol condensation of 4-Methoxybenzaldehyde and Acetone, will be synthesized in a one pot reaction. ...
Chapter 20 reactions of carbonyls
... • Carbonyl compounds that also contain N–H or O–H bonds undergo an acid–base reaction with organometallic reagents, not nucleophilic addition. ...
... • Carbonyl compounds that also contain N–H or O–H bonds undergo an acid–base reaction with organometallic reagents, not nucleophilic addition. ...
7. Alkenes: Reactions and Synthesis
... molecules, formed by polymerization Alkenes react with ...
... molecules, formed by polymerization Alkenes react with ...
Functional Groups - La Salle University
... oxycontin (slow release) percocet (w/ acetominophen) ...
... oxycontin (slow release) percocet (w/ acetominophen) ...
Organic Chemistry I (CHEM 2010 and 2012)
... expected to read and study the material to be discussed prior to the lecture. This includes working in-chapter and end-of-chapter problems and exercises in the text. Students should review the material discussed until comprehension is acquired and seek assistance when necessary. It is also highly re ...
... expected to read and study the material to be discussed prior to the lecture. This includes working in-chapter and end-of-chapter problems and exercises in the text. Students should review the material discussed until comprehension is acquired and seek assistance when necessary. It is also highly re ...
Experiment 7-Reduction
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
... A reduction is often defined as the gain of two hydrogen atoms or the loss of an oxygen atom, or both. This leads to a very important conversion reaction, where aldehydes and ketones are reduced to primary and secondary alcohols. O ...
11 - MSU Chemistry
... Solutions for Chapter 11 – Nucleophilic Substitution at C=O with Loss of Oxygen ...
... Solutions for Chapter 11 – Nucleophilic Substitution at C=O with Loss of Oxygen ...
unit 17 organic compounds containing oxygen and nitrogen atoms
... synthetic organic chemistry as a means of carbonyl group protection. In some chemical reactions one functional group may interfere with intended reaction elsewhere in a complex molecule. We can often circumvent the problem in such cases by first protecting the interfering functional group, carrying ...
... synthetic organic chemistry as a means of carbonyl group protection. In some chemical reactions one functional group may interfere with intended reaction elsewhere in a complex molecule. We can often circumvent the problem in such cases by first protecting the interfering functional group, carrying ...
EXPERIMENT 6: Reactions of Carbonyl Compounds: Qualitative
... steam distillation in the presence of dilute acid. The most commonly used for identification are the 2,4-dinitrophenylhydrazones since simple carbonyl compounds give very colourful, highly crystalline solids. These solid derivatives can also be prepared very rapidly, which makes them an extremely us ...
... steam distillation in the presence of dilute acid. The most commonly used for identification are the 2,4-dinitrophenylhydrazones since simple carbonyl compounds give very colourful, highly crystalline solids. These solid derivatives can also be prepared very rapidly, which makes them an extremely us ...
Overview of the Reactions of Carbonyl Compounds
... nucleophilic addition reaction but the mechanisms for both reactions involves the same 1st step. • In this step, the nucleophile bonds to the carbonyl carbon and thereby causes a carbon-oxygen bond to break. The carbonyl carbon rehybridizes from sp2 to sp3 and the carbonyl oxygen becomes negatively ...
... nucleophilic addition reaction but the mechanisms for both reactions involves the same 1st step. • In this step, the nucleophile bonds to the carbonyl carbon and thereby causes a carbon-oxygen bond to break. The carbonyl carbon rehybridizes from sp2 to sp3 and the carbonyl oxygen becomes negatively ...
Abstracts - Thieme Verlag
... This chapter provides a concise overview of metal-catalyzed additions to alkynes that involve carbon monoxide and a nucleophilic species, such as water, an alcohol, a thiol, or an amine. Alkynes undergo very efficient hydroalkoxyesterifications of the type extensively studied in alkene carbonylation ...
... This chapter provides a concise overview of metal-catalyzed additions to alkynes that involve carbon monoxide and a nucleophilic species, such as water, an alcohol, a thiol, or an amine. Alkynes undergo very efficient hydroalkoxyesterifications of the type extensively studied in alkene carbonylation ...
Παρουσίαση του PowerPoint
... Before and during these syntheses, groups of chemists sitting around blackboards or piles of paper plan the work they are about to undertake. Possible routes are drawn out, criticized, modified again when the behavior of the compounds in the flask turns out to be different from what was expected, un ...
... Before and during these syntheses, groups of chemists sitting around blackboards or piles of paper plan the work they are about to undertake. Possible routes are drawn out, criticized, modified again when the behavior of the compounds in the flask turns out to be different from what was expected, un ...
Topics 10 and 20 Outline
... • SN2 reactions are best conducted using aprotic, polar solvents and SN1 reactions are best conducted using protic, polar solvents. Electrophilic Addition Reactions: • An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids. • ...
... • SN2 reactions are best conducted using aprotic, polar solvents and SN1 reactions are best conducted using protic, polar solvents. Electrophilic Addition Reactions: • An electrophile is an electron-deficient species that can accept electron pairs from a nucleophile. Electrophiles are Lewis acids. • ...























