CHEMISTRY CHAPTER 5 OUTLINE NOTES 5.1 – Light and
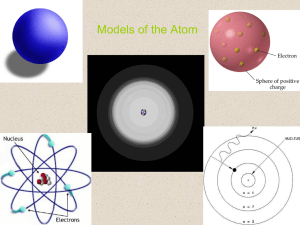
... 5.1 – Light and Quantized Energy • The Nuclear Atom and Unanswered Questions o Although Rutherford’s scientific model of an atom was a breakthrough, it lacked detail about how electrons occupy the space surrounding the nucleus of an atom. o Questions Still Unanswered: • How are an atom’s electrons a ...
... 5.1 – Light and Quantized Energy • The Nuclear Atom and Unanswered Questions o Although Rutherford’s scientific model of an atom was a breakthrough, it lacked detail about how electrons occupy the space surrounding the nucleus of an atom. o Questions Still Unanswered: • How are an atom’s electrons a ...
Figure 2: Alternative Periodic Table
... K4+ -> K5+ + eb) Explain the large jumps in ionization energy between the 9th and the 10th and the 17th and the 18th. The jump between the 9th and 10th represents a change from ionizing n=3 electron to ionizing n=2 electrons. The jump between the 17th and 18th represents a change from ionizing n=2 e ...
... K4+ -> K5+ + eb) Explain the large jumps in ionization energy between the 9th and the 10th and the 17th and the 18th. The jump between the 9th and 10th represents a change from ionizing n=3 electron to ionizing n=2 electrons. The jump between the 17th and 18th represents a change from ionizing n=2 e ...
collective states of 2d electron-hole system under the influence of
... energy of the metallic-type electron–hole liquid is investigated in the Hartree–Fock approximation. We have established that chemical potential is monotonic function versus the value of the filling factor with negative compressibility, which leads to instability of the Bose-Einstein condensate of ma ...
... energy of the metallic-type electron–hole liquid is investigated in the Hartree–Fock approximation. We have established that chemical potential is monotonic function versus the value of the filling factor with negative compressibility, which leads to instability of the Bose-Einstein condensate of ma ...
key
... K4+ -> K5+ + eb) Explain the large jumps in ionization energy between the 9th and the 10th and the 17th and the 18th. The jump between the 9th and 10th represents a change from ionizing n=3 electron to ionizing n=2 electrons. The jump between the 17th and 18th represents a change from ionizing n=2 e ...
... K4+ -> K5+ + eb) Explain the large jumps in ionization energy between the 9th and the 10th and the 17th and the 18th. The jump between the 9th and 10th represents a change from ionizing n=3 electron to ionizing n=2 electrons. The jump between the 17th and 18th represents a change from ionizing n=2 e ...
Chemical Bonding
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
Chemical Bonding
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
Unit Map Chemistry I Unit IV
... Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per sublevel, and the number of orbitals per main energy level. List the total number of electrons needed to fully occupy each main energy level. State the Aufbau principle, the Pauli exclusi ...
... Relate the number of sublevels corresponding to each of an atom’s main energy levels, the number of orbitals per sublevel, and the number of orbitals per main energy level. List the total number of electrons needed to fully occupy each main energy level. State the Aufbau principle, the Pauli exclusi ...
File
... Electrons will occupy orbitals having the lowest energy FIRST and then in order of increasing energy. The "Ground State" of an atom is when every electron is in its lowest energy ...
... Electrons will occupy orbitals having the lowest energy FIRST and then in order of increasing energy. The "Ground State" of an atom is when every electron is in its lowest energy ...
Atomic Variational Calculations: Hydrogen to Boron
... orthonormal hydrogenic wave functions shown below will be used. The method will be illustrated for boron, but can be used for any atomic or ionic species with five or less electrons. Using the following orthonormal trial wave functions, the various contributions to the total electronic energy of a m ...
... orthonormal hydrogenic wave functions shown below will be used. The method will be illustrated for boron, but can be used for any atomic or ionic species with five or less electrons. Using the following orthonormal trial wave functions, the various contributions to the total electronic energy of a m ...
Electrons in Atoms
... Like Bohr’s model, electrons are restricted to certain energy levels Unlike Bohr’s model, the exact pathway of the electron is uncertain Locations of electrons are uncertain, and described terms of probability…. i.e. the likelihood of finding the electron at a given point in time ...
... Like Bohr’s model, electrons are restricted to certain energy levels Unlike Bohr’s model, the exact pathway of the electron is uncertain Locations of electrons are uncertain, and described terms of probability…. i.e. the likelihood of finding the electron at a given point in time ...
Semester Exam Practice Questions
... 48. The formula mass of magnesium chloride, MgCl2, is __________. a. 59.8 amu c. 95.2 amu b. 76.4 amu d. 125.8 amu 49. If one molecule of NH3 has a mass of 17.0 g/mol, what is the mass of 6.02 x 1023 molecules of NH3? a. 2.82 g c. 102 g b. 17.0 g d. 2.82 x 10-25 g 50. Which of the following statemen ...
... 48. The formula mass of magnesium chloride, MgCl2, is __________. a. 59.8 amu c. 95.2 amu b. 76.4 amu d. 125.8 amu 49. If one molecule of NH3 has a mass of 17.0 g/mol, what is the mass of 6.02 x 1023 molecules of NH3? a. 2.82 g c. 102 g b. 17.0 g d. 2.82 x 10-25 g 50. Which of the following statemen ...
Chemistry - nyostrander.us
... element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. According to the wave-mechanical model, an orbital is defined as the (1) circular path for electrons ...
... element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. According to the wave-mechanical model, an orbital is defined as the (1) circular path for electrons ...
Chem 1a Midterm Review
... not completely shield the added positive charge thus the effective nuclear charge goes up and the electrons are held more tightly, Iz goes up, and the atom becomes smaller. When you add an electron to a completely empty orbital of a larger n the size of the atom gets much larger and the Iz will go d ...
... not completely shield the added positive charge thus the effective nuclear charge goes up and the electrons are held more tightly, Iz goes up, and the atom becomes smaller. When you add an electron to a completely empty orbital of a larger n the size of the atom gets much larger and the Iz will go d ...
Modern Model of the Atom
... 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE - when electrons occupy orbitals of equal energy one electron enters each orbital until al ...
... 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic orbital may hold at most two electrons. Each must have an opposite spin. 3. HUND’S RULE - when electrons occupy orbitals of equal energy one electron enters each orbital until al ...
Electrons in Atoms
... In most natural phenomena, change trends toward lower energy Systems are more stable when they have less energy. Electrons also tend to arrange themselves in their lowest energy states. The arrangement of electrons within an atom is called an electron configuration. ...
... In most natural phenomena, change trends toward lower energy Systems are more stable when they have less energy. Electrons also tend to arrange themselves in their lowest energy states. The arrangement of electrons within an atom is called an electron configuration. ...
Ch. 4-2 PowerPoint
... Higher density – more likely to find electron Lower density – less likely to find electron ...
... Higher density – more likely to find electron Lower density – less likely to find electron ...
ExamView - Untitled.tst
... a. the atom becomes charged. b. the atom becomes unstable. c. the electron’s location is pinpointed. d. the atom gains or loses energy. ____ 17. Which of the following statements about the modern model of the atom is true? a. Electrons can be found between energy levels. b. It is possible to find el ...
... a. the atom becomes charged. b. the atom becomes unstable. c. the electron’s location is pinpointed. d. the atom gains or loses energy. ____ 17. Which of the following statements about the modern model of the atom is true? a. Electrons can be found between energy levels. b. It is possible to find el ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.