Abstract
... electrons in the CB gain energy until they reach a threshold kinetic energy equal to the bandgap. These excited electrons then may collide with valence electrons and promote them into the CB by energy exchange. In order to reach this threshold energy for collisional ionization, conduction electrons ...
... electrons in the CB gain energy until they reach a threshold kinetic energy equal to the bandgap. These excited electrons then may collide with valence electrons and promote them into the CB by energy exchange. In order to reach this threshold energy for collisional ionization, conduction electrons ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... 3. During a flame test, a lithium salt produces a characteristic red flame. This red color is produced when electrons in excited lithium atoms A. are lost by the atoms. B. are gained by the atoms. C. return to lower energy states within the atoms. D. move to higher energy states within the atoms. (N ...
... 3. During a flame test, a lithium salt produces a characteristic red flame. This red color is produced when electrons in excited lithium atoms A. are lost by the atoms. B. are gained by the atoms. C. return to lower energy states within the atoms. D. move to higher energy states within the atoms. (N ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... 3. During a flame test, a lithium salt produces a characteristic red flame. This red color is produced when electrons in excited lithium atoms A. are lost by the atoms. B. are gained by the atoms. C. return to lower energy states within the atoms. D. move to higher energy states within the atoms. (N ...
... 3. During a flame test, a lithium salt produces a characteristic red flame. This red color is produced when electrons in excited lithium atoms A. are lost by the atoms. B. are gained by the atoms. C. return to lower energy states within the atoms. D. move to higher energy states within the atoms. (N ...
Electromagnetic Radiation
... Electron Configuration & Magnetic Properties •Diamagnetic materials have all electrons paired and are not attracted to a magnetic ...
... Electron Configuration & Magnetic Properties •Diamagnetic materials have all electrons paired and are not attracted to a magnetic ...
Chapter 9: Chemical Quantities
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
... - Emission and Absorption of Light by atoms and possible transitions of electrons ...
chem 1411- chapter 7
... unstable. The electrons then start falling from higher levels to lower levels, releasing energy. This energy when resolved through a spectroscope, we get different lines of specific wavelengths. This is called Atomic Emission Spectrum or the Line Spectrum. This is a discontinuous spectrum. When radi ...
... unstable. The electrons then start falling from higher levels to lower levels, releasing energy. This energy when resolved through a spectroscope, we get different lines of specific wavelengths. This is called Atomic Emission Spectrum or the Line Spectrum. This is a discontinuous spectrum. When radi ...
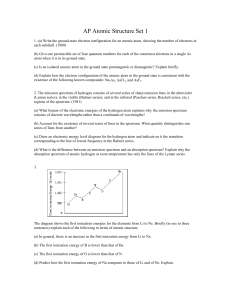
AP Atomic Structure Set 1
... (b) Account for the existence of several series of lines in the spectrum. What quantity distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate on it the transition corresponding to the line of lowest frequency in the Balmer seri ...
... (b) Account for the existence of several series of lines in the spectrum. What quantity distinguishes one series of lines from another? (c) Draw an electronic energy level diagram for the hydrogen atom and indicate on it the transition corresponding to the line of lowest frequency in the Balmer seri ...
Spin Polarized Electron - Jordan University of Science and
... Historically, the development of a suitable SPE source and have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) Photoemission from polarized atoms 3) Fano effect 4) The most suitable source photoemission from GaAs. ...
... Historically, the development of a suitable SPE source and have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) Photoemission from polarized atoms 3) Fano effect 4) The most suitable source photoemission from GaAs. ...
Problem Set 11: Chemistry Graduate Quantum I Physics 6572
... third 2p electron from the nucleus. This emitted particle is called an Auger electron. ...
... third 2p electron from the nucleus. This emitted particle is called an Auger electron. ...
Grade 9 Chemistry Unit Test Name: Part A: Multiple Choice (15
... d) Antoine Lavoisier _____ 2. Which group of “scientists” was very hands-on, but also very secretive? a) Francis Bacon b) Joseph Proust c) The Alchemists d) Antoine Lavoisier _____ 3. Which scientist first defined elements as pure substances and identified 23 new elements? a) Francis Bacon b) Joseph ...
... d) Antoine Lavoisier _____ 2. Which group of “scientists” was very hands-on, but also very secretive? a) Francis Bacon b) Joseph Proust c) The Alchemists d) Antoine Lavoisier _____ 3. Which scientist first defined elements as pure substances and identified 23 new elements? a) Francis Bacon b) Joseph ...
5 - BrainMass
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
... smaller than that for a 3p electron. In light of this fact, which orbital is higher in energy? b. Would you expect it to require more or less energy to remove a 3s electron from the chlorine atom, as compared with a 2p electron? Explain. ...
Measurement of the half-life of
... the AVF cyclotron at Research Center for Nuclear Physics, Osaka University. The cyclotron was operated at an α-particle energy of 40 MeV. The Ti target was placed in close contact with an aluminium degrader to adjust the alpha-particle energy to 30 MeV in the middle of target. After the 30 min irrad ...
... the AVF cyclotron at Research Center for Nuclear Physics, Osaka University. The cyclotron was operated at an α-particle energy of 40 MeV. The Ti target was placed in close contact with an aluminium degrader to adjust the alpha-particle energy to 30 MeV in the middle of target. After the 30 min irrad ...
NAME PERIOD ______ DATE Chapter 5 Sec. 2
... 2. What do the sublevel designations s, p, d, and f specify with respect to the atom’s orbitals? ...
... 2. What do the sublevel designations s, p, d, and f specify with respect to the atom’s orbitals? ...
Problem Set 1 (Due January 30th by 7:00 PM) Answers to the
... Energy, Electrons and Periodic Trends (Bonus Date: January 26th) For all periodic trend problems, make sure that you can justify your answer. You will be expected to do this on an exam. 14. For each group of atoms, determine which would have a higher 1st Ionization Energy. a. Xe, Kr, Ar b. As, Cl, B ...
... Energy, Electrons and Periodic Trends (Bonus Date: January 26th) For all periodic trend problems, make sure that you can justify your answer. You will be expected to do this on an exam. 14. For each group of atoms, determine which would have a higher 1st Ionization Energy. a. Xe, Kr, Ar b. As, Cl, B ...
4.quantumorbitals
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
... Quantum Theory The electron is like a cloud of negative energy or a wave. Orbitals are areas in 3D space where the electrons most probably are. The energy of the electron is in its vibrational modes- like notes on a guitar string. Photons are produced when high energy modes change to lower energy mo ...
Solution
... For the following ten questions, consider the lowest energy Lewis structure (minimized formal charge etc.) for the following molecules/ions: XeF4, XeO4, OCN–1 (you may want to draw the Lewis structures in the space provided, the central atom is highlighted). ...
... For the following ten questions, consider the lowest energy Lewis structure (minimized formal charge etc.) for the following molecules/ions: XeF4, XeO4, OCN–1 (you may want to draw the Lewis structures in the space provided, the central atom is highlighted). ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.