1. Conduction electrons in a metal: the free
... The inner-shell electrons are hardly affected. Conversely, the outer electrons - the valence electrons - can be said to move away from their respective atoms and move about like a more-or-less homogeneous electron gas. The atomic nuclei and the inner electrons, which remain in approximately unchange ...
... The inner-shell electrons are hardly affected. Conversely, the outer electrons - the valence electrons - can be said to move away from their respective atoms and move about like a more-or-less homogeneous electron gas. The atomic nuclei and the inner electrons, which remain in approximately unchange ...
cond-mat/0205001 PDF
... shown with arrows. We explain how we obtain the field Bc ∼ 0.011 T later in the text. The crossover is seen more clearly in the inset of the figure where we show a blow up of the same graph at small fields. The field Bc corresponds to ∼ 30% of the theoretical value 1/µ0 ∼ 0.036 T given for no e-e br ...
... shown with arrows. We explain how we obtain the field Bc ∼ 0.011 T later in the text. The crossover is seen more clearly in the inset of the figure where we show a blow up of the same graph at small fields. The field Bc corresponds to ∼ 30% of the theoretical value 1/µ0 ∼ 0.036 T given for no e-e br ...
Bonding and Structure Organic Molecular Structure
... • Filled shell or Octet rule doesn't really work for 3rd row elements, e.g. phosphorus and sulfur, see why later • Obeying the filled shell "rule" is the same as obeying the normal RULES OF VALENCE (normal number of bands), i.e. 4 bonds to each C, 3 bonds to each N, 2 bonds to each O etc. Example Pr ...
... • Filled shell or Octet rule doesn't really work for 3rd row elements, e.g. phosphorus and sulfur, see why later • Obeying the filled shell "rule" is the same as obeying the normal RULES OF VALENCE (normal number of bands), i.e. 4 bonds to each C, 3 bonds to each N, 2 bonds to each O etc. Example Pr ...
Structural Analysis of Nanostructures with Electron Microscopy 1
... perfectly simultaneous. Often it is helpful to compare the pictures obtained with both techniques to get more information about the specimen. The resolutions achievable with these techniques are between 0.2 and 0.5 nm(5) . ...
... perfectly simultaneous. Often it is helpful to compare the pictures obtained with both techniques to get more information about the specimen. The resolutions achievable with these techniques are between 0.2 and 0.5 nm(5) . ...
1 Introduction - High Point University
... Because the states an electron occur only at discrete energy levels, they are said to be quantized. The word quantum comes from a Latin word meaning “how much.” The branch of physics that provides the current model of the Hydrogen atom is called quantum mechanics. The electron in a Hydrogen atom can ...
... Because the states an electron occur only at discrete energy levels, they are said to be quantized. The word quantum comes from a Latin word meaning “how much.” The branch of physics that provides the current model of the Hydrogen atom is called quantum mechanics. The electron in a Hydrogen atom can ...
Doublet Fine Structure and the Spinning Electron
... From t he very earli est obser va ti ons of spectra l series it has been known t hat each member of certain genera l t y pes of series shows fine st ruc t ur e while t hose of others do not. Each member of some of t he series in the alkali metals, for example, is a close doublet (see F ig. 1.9), whe ...
... From t he very earli est obser va ti ons of spectra l series it has been known t hat each member of certain genera l t y pes of series shows fine st ruc t ur e while t hose of others do not. Each member of some of t he series in the alkali metals, for example, is a close doublet (see F ig. 1.9), whe ...
Observation of a collimated bunch of high
... allowed us to verify that the experimental conditions required for the growth of high amplitude relativistic plasma waves in the wake of a super-intense 35 fs laser pulse were indeed achieved. We measured the angular distribution and energy spectrum of accelerated electrons. We also found that, movi ...
... allowed us to verify that the experimental conditions required for the growth of high amplitude relativistic plasma waves in the wake of a super-intense 35 fs laser pulse were indeed achieved. We measured the angular distribution and energy spectrum of accelerated electrons. We also found that, movi ...
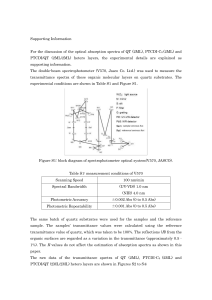
Supporting Information For the discussion of the optical absorption
... The same batch of quartz substrates were used for the samples and the reference sample. The samples’ transmittance values were calculated using the reference transmittance value of quartz, which was taken to be 100%. The reflections (R) from the organic surfaces are regarded as a variation in the tr ...
... The same batch of quartz substrates were used for the samples and the reference sample. The samples’ transmittance values were calculated using the reference transmittance value of quartz, which was taken to be 100%. The reflections (R) from the organic surfaces are regarded as a variation in the tr ...
Spin-based quantum computing using electrons on liquid helium
... exceptionally high vacuum is attained at mK temperatures, and thus we expect few impurities to accumulate. Paramagnetic defects in the electrode structure and its supports will contribute to decoherence. It is difficult to estimate the quantity and effect of these defects. Such defects will have the ...
... exceptionally high vacuum is attained at mK temperatures, and thus we expect few impurities to accumulate. Paramagnetic defects in the electrode structure and its supports will contribute to decoherence. It is difficult to estimate the quantity and effect of these defects. Such defects will have the ...
Title Goes Here
... other words, we need to take into account Coulomb interactions between conduction electrons and a valence hole to explain these bound complexes. In 1D systems, Coulomb interactions, or excitonic effects, become strong due to its large quantum confinement [15]. However, it has not been fully understo ...
... other words, we need to take into account Coulomb interactions between conduction electrons and a valence hole to explain these bound complexes. In 1D systems, Coulomb interactions, or excitonic effects, become strong due to its large quantum confinement [15]. However, it has not been fully understo ...
Hund`s Rule for Composite Fermions
... has only two states 0-1 and 1-0. Thus, the CF theory predicts that the lowest two states of (6| 72 ) are 1-0 and 0-1, and the next state is 2-1, in agreement with the exact spectrum of Fig.1. The CF wave functions [16] have overlaps of 0.9959, 0.9978, and 0.9970 with the exact 0-1, 1-0, and 2-1 sta ...
... has only two states 0-1 and 1-0. Thus, the CF theory predicts that the lowest two states of (6| 72 ) are 1-0 and 0-1, and the next state is 2-1, in agreement with the exact spectrum of Fig.1. The CF wave functions [16] have overlaps of 0.9959, 0.9978, and 0.9970 with the exact 0-1, 1-0, and 2-1 sta ...
atomic spectroscopy 2005
... momentum for the state: L = l(l +1) h . The magnetic quantum number, m, specifies the projection of the total angular momentum on a given axis: Lz = mh , with m = !l, ! l + 1, .... , l ! 1, l In the absence of an external magnetic field, we do not expect the energy of the state to depend on its orie ...
... momentum for the state: L = l(l +1) h . The magnetic quantum number, m, specifies the projection of the total angular momentum on a given axis: Lz = mh , with m = !l, ! l + 1, .... , l ! 1, l In the absence of an external magnetic field, we do not expect the energy of the state to depend on its orie ...
Exploring Compton Scattering Using the Spectrum
... The maximum change in wavelength for the photon happens when it transfers as much momentum as possible to the electron. That is, when cos(θ)=-1. The maximum change in wavelength is thus: ...
... The maximum change in wavelength for the photon happens when it transfers as much momentum as possible to the electron. That is, when cos(θ)=-1. The maximum change in wavelength is thus: ...
Spectroscopic Notation Most of the information we have about the
... us via emission lines. However, before we can begin to understand how emission lines are formed, and what they tell us, we must first go over some basic notation and review some atomic physics. We will begin with spectroscopic notation. Electrons are described with 4 quantum numbers: the principle q ...
... us via emission lines. However, before we can begin to understand how emission lines are formed, and what they tell us, we must first go over some basic notation and review some atomic physics. We will begin with spectroscopic notation. Electrons are described with 4 quantum numbers: the principle q ...
On the Nature of the Change in the Wave Function in a
... 1 or hole 2 is not a common factor. It should be remembered that some causative factor is implied by the very different electron distributions in Gedankenexperiments 1 and 2. It is reasonable to conclude that knowledge by the observer regarding the particular path of the electron through the wall is ...
... 1 or hole 2 is not a common factor. It should be remembered that some causative factor is implied by the very different electron distributions in Gedankenexperiments 1 and 2. It is reasonable to conclude that knowledge by the observer regarding the particular path of the electron through the wall is ...
Wave Nature of Light
... • Electrons dropping from the third orbit to the second orbit cause the red line. • Note that electron transitions from higherenergy orbits to the second orbit account for all of hydrogen’s visible lines. ...
... • Electrons dropping from the third orbit to the second orbit cause the red line. • Note that electron transitions from higherenergy orbits to the second orbit account for all of hydrogen’s visible lines. ...
Remarks on energetic conditions for positronium formation in non
... The interaction of positron with matter is a subject of radiation physics and chemistry [1, 2]. However, in this case, the energetic positron is subjected. At this scale of energy, the interactions resemble these for an electron – both particles can be classified as light charged particles. When thes ...
... The interaction of positron with matter is a subject of radiation physics and chemistry [1, 2]. However, in this case, the energetic positron is subjected. At this scale of energy, the interactions resemble these for an electron – both particles can be classified as light charged particles. When thes ...
Master thesis Single photon double valence ionization of
... The orbitals get their names from the principal and angular momentum quantum numbers. The names s-, p-, d- and f-orbitals are referred to the orbitals with the angular momentum quantum numbers l = 0, 1, 2, and 3 respectively. These labels together with the principal quantum number are usually used t ...
... The orbitals get their names from the principal and angular momentum quantum numbers. The names s-, p-, d- and f-orbitals are referred to the orbitals with the angular momentum quantum numbers l = 0, 1, 2, and 3 respectively. These labels together with the principal quantum number are usually used t ...
Experiment Note - Spectrum Techniques
... photon’s frequency f (or, conversely by an increase in its wavelength λ) since E=hf. As we can see, the photon loses some energy after the collision, bouncing off at an angle θ with a new energy E’ and momentum p’, which translate into a decrease in frequency to f’. Of course, the initial photon’s w ...
... photon’s frequency f (or, conversely by an increase in its wavelength λ) since E=hf. As we can see, the photon loses some energy after the collision, bouncing off at an angle θ with a new energy E’ and momentum p’, which translate into a decrease in frequency to f’. Of course, the initial photon’s w ...
Dissociation energy of the C-H bond in chloroform Cl3C
... quantum number. For a peak observed at wavenumber , the transition energy is Δ E=hc ~ where h is Planck's constant and c is the speed of light. The energy calculated in that way is energy per photon, and may be converted to energy per mole by multiplying by Avogadro's number. For a peak observed ...
... quantum number. For a peak observed at wavenumber , the transition energy is Δ E=hc ~ where h is Planck's constant and c is the speed of light. The energy calculated in that way is energy per photon, and may be converted to energy per mole by multiplying by Avogadro's number. For a peak observed ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.