Electrodynamics of Solids
... the ordered state has a broken symmetry, hence the name broken symmetry states of metals. For these states, which are called charge or spin density wave states, translational symmetry is broken and the electronic charge or spin density assumes a periodic variation – much like the periodic arrangemen ...
... the ordered state has a broken symmetry, hence the name broken symmetry states of metals. For these states, which are called charge or spin density wave states, translational symmetry is broken and the electronic charge or spin density assumes a periodic variation – much like the periodic arrangemen ...
Chemistry Handout 08 - (Redox)
... gain electrons and have an increase in oxidation number lose electrons and have an increase in oxidation number gain electrons and have a decrease in oxidation number lose electrons and have a decrease in oxidation number ...
... gain electrons and have an increase in oxidation number lose electrons and have an increase in oxidation number gain electrons and have a decrease in oxidation number lose electrons and have a decrease in oxidation number ...
Unit 3 Review Questions - Unit #1-0
... 5. In metallic bonding, the valence electrons of all atoms are shared in: 1. ? a nonpolar covalent bond 2. ? an electron sea 3. ? transferred to nonmetallic ions 4. ? a polar covalent bond ...
... 5. In metallic bonding, the valence electrons of all atoms are shared in: 1. ? a nonpolar covalent bond 2. ? an electron sea 3. ? transferred to nonmetallic ions 4. ? a polar covalent bond ...
Study of excited states of fluorinated copper phthalocyanine by inner
... Figure 1a and b show carbon (C) K-edge NEXAFS spectra of FCuPc at α = 0◦ (normal incidence), 35, 55, and 70◦ (grazing incidence). Four sharp peaks at hν = 284.6, 285.5, 287.8, and 289.5 eV and one broad peak at about 295 eV appear at grazing incidence. The first three peaks show strong polarization ...
... Figure 1a and b show carbon (C) K-edge NEXAFS spectra of FCuPc at α = 0◦ (normal incidence), 35, 55, and 70◦ (grazing incidence). Four sharp peaks at hν = 284.6, 285.5, 287.8, and 289.5 eV and one broad peak at about 295 eV appear at grazing incidence. The first three peaks show strong polarization ...
Problems
... Always provide the analytical answer before finding numerical values. Please print out the question sheet(s) and staple to the top of your homework. Write your name, email address, and date/time the assignment is turned in on the cover. Assignments must be turned in before class on the due date. The ...
... Always provide the analytical answer before finding numerical values. Please print out the question sheet(s) and staple to the top of your homework. Write your name, email address, and date/time the assignment is turned in on the cover. Assignments must be turned in before class on the due date. The ...
Plasmon electron energy-gain spectroscopy
... than the de Broglie wavelength at typical electron-beam energies ⇠200 keV. The analysis of energy exchanges between the electrons and the sample adds further information on the chemical composition and electronic structure of the specimen with similar spatial resolution. In particular, electron ener ...
... than the de Broglie wavelength at typical electron-beam energies ⇠200 keV. The analysis of energy exchanges between the electrons and the sample adds further information on the chemical composition and electronic structure of the specimen with similar spatial resolution. In particular, electron ener ...
Final Exam A - Answers - San Diego Chemistry Tutor
... bond energy of 467 kJ/mol. Listed below are some lasers, which is the least expensive laser, that provides enough energy to be able to break O-H bonds? a) 157 nm ($35,000) b) 250 nm ($10,000) c) 405 nm ($5,000) d) 488 nm ($2,500) e) None of these lasers have enough energy. 12. A hydrogen atom has i ...
... bond energy of 467 kJ/mol. Listed below are some lasers, which is the least expensive laser, that provides enough energy to be able to break O-H bonds? a) 157 nm ($35,000) b) 250 nm ($10,000) c) 405 nm ($5,000) d) 488 nm ($2,500) e) None of these lasers have enough energy. 12. A hydrogen atom has i ...
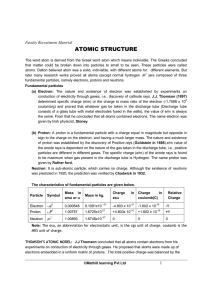
ATOMIC STRUCTURE 2.1 THE ATOM
... has 82 protons and (207–82) 125 neutrons (i.e. the p:n ratio is approximately 2:3). In order to preserve electrical neutrality, the number of electrons in an atom is equal to the number of protons, so that aluminium has 13 electrons, which exist outside of the nucleus in shells of differing energies ...
... has 82 protons and (207–82) 125 neutrons (i.e. the p:n ratio is approximately 2:3). In order to preserve electrical neutrality, the number of electrons in an atom is equal to the number of protons, so that aluminium has 13 electrons, which exist outside of the nucleus in shells of differing energies ...
Molecular Term Symbols
... The Franck-Condon factor shows, the probability of a vibrationalelectronic transition is governed by the overlap between the final and initial vibrational wave functions at fixed internuclear distances. Moreover, in the simplest sense, we can take the initial states to be ground molecular electronic ...
... The Franck-Condon factor shows, the probability of a vibrationalelectronic transition is governed by the overlap between the final and initial vibrational wave functions at fixed internuclear distances. Moreover, in the simplest sense, we can take the initial states to be ground molecular electronic ...
2.2.3.- X-ray diffraction
... Laue who in 1912 demonstrated that x-rays could have a comparable wavelength to the atomic spacing in crystals and, therefore, they could be diffracted [36]. This was immediately confirmed by Walter Friedrich and Paul Knipping [36]. In 1914 Darwin elaborated a Kinematic Theory of Diffraction, which ...
... Laue who in 1912 demonstrated that x-rays could have a comparable wavelength to the atomic spacing in crystals and, therefore, they could be diffracted [36]. This was immediately confirmed by Walter Friedrich and Paul Knipping [36]. In 1914 Darwin elaborated a Kinematic Theory of Diffraction, which ...
atomic structure 2.1 the atom - Aula Virtual Maristas Mediterránea
... has 82 protons and (207–82) 125 neutrons (i.e. the p:n ratio is approximately 2:3). In order to preserve electrical neutrality, the number of electrons in an atom is equal to the number of protons, so that aluminium has 13 electrons, which exist outside of the nucleus in shells of differing energies ...
... has 82 protons and (207–82) 125 neutrons (i.e. the p:n ratio is approximately 2:3). In order to preserve electrical neutrality, the number of electrons in an atom is equal to the number of protons, so that aluminium has 13 electrons, which exist outside of the nucleus in shells of differing energies ...
EDS system
... within the detector whilst being as transparent as possible to low energy X-rays. • There are two main types of window materials. • Beryllium (Be) is highly robust, but strongly absorbs low energy X-rays meaning that only elements from sodium (Na) can be detected. • Polymer-based thin windows can be ...
... within the detector whilst being as transparent as possible to low energy X-rays. • There are two main types of window materials. • Beryllium (Be) is highly robust, but strongly absorbs low energy X-rays meaning that only elements from sodium (Na) can be detected. • Polymer-based thin windows can be ...
Urban - TEM aberration correction review
... there are two major differences Fig. 3. (A) Transversal inversion polarization-domain wall in ferroelectric PZT. of atomic structure enabled the that are decisive for image for- Arrows give the direction of the spontaneous polarization, which can be directly discovery of a new type of reconmation in ...
... there are two major differences Fig. 3. (A) Transversal inversion polarization-domain wall in ferroelectric PZT. of atomic structure enabled the that are decisive for image for- Arrows give the direction of the spontaneous polarization, which can be directly discovery of a new type of reconmation in ...
Chapter 6 Electronic Structure of Atoms
... Explanation: The de Broglie wavelength of an object can be calculated by remembering that 1 J = 1 kg•m2•s-2, which changes h = 6.626 x 10-34 kg •m2•s-1. Now using the formula that relates the mass and velocity of an object to its wavelength, the wavelength can be calculated as follows: ...
... Explanation: The de Broglie wavelength of an object can be calculated by remembering that 1 J = 1 kg•m2•s-2, which changes h = 6.626 x 10-34 kg •m2•s-1. Now using the formula that relates the mass and velocity of an object to its wavelength, the wavelength can be calculated as follows: ...
Single-Electron Capacitance Spectroscopy R. Ashoori Optics and Devices
... The principle focus of research in our laboratory lies in the study of interacting electronic systems in low dimensional semiconductor structures. Systems in which electrons exist purely in two or one dimensions and even small boxes (quantum dots) containing as few as one electron can now be produce ...
... The principle focus of research in our laboratory lies in the study of interacting electronic systems in low dimensional semiconductor structures. Systems in which electrons exist purely in two or one dimensions and even small boxes (quantum dots) containing as few as one electron can now be produce ...
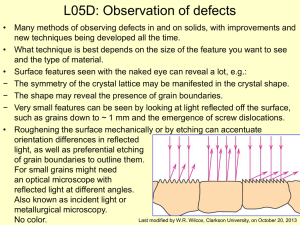
L05D - Clarkson University
... http://www.youtube.com/watch?feature=player_embedded&v=oSCX78-8-q0 ...
... http://www.youtube.com/watch?feature=player_embedded&v=oSCX78-8-q0 ...
Atomic Structure Notes
... There are various trends in this graph which can be explained by reference to the proton number and electronic configuration of the various elements. A number of factors must be considered: - Energy is required to remove electrons from atoms in order to overcome their attraction to the nucleus. The ...
... There are various trends in this graph which can be explained by reference to the proton number and electronic configuration of the various elements. A number of factors must be considered: - Energy is required to remove electrons from atoms in order to overcome their attraction to the nucleus. The ...
Chapter 3
... Calculate the energy (in joules) of (a) a photon with a wavelength of 5.00×104 nm (infrared region) and (b) a photon with a wavelength of 52 nm (ultraviolet region). (c) Calculate the maximum kinetic energy of an electron ejected by the photon in part (b) from a metal with a binding energy of 3.7 eV ...
... Calculate the energy (in joules) of (a) a photon with a wavelength of 5.00×104 nm (infrared region) and (b) a photon with a wavelength of 52 nm (ultraviolet region). (c) Calculate the maximum kinetic energy of an electron ejected by the photon in part (b) from a metal with a binding energy of 3.7 eV ...
Helium atom in metallic electron gases: A comparative study
... same product form of 1s-type parametric hydrogenic functions to perform exploratory variational calculations. Both models employ induced charge densities in the corresponding Poisson’s equations with a fixed point-like nucleus, but the underlying charge-density response of the host system is generat ...
... same product form of 1s-type parametric hydrogenic functions to perform exploratory variational calculations. Both models employ induced charge densities in the corresponding Poisson’s equations with a fixed point-like nucleus, but the underlying charge-density response of the host system is generat ...
Auger electron spectroscopy
.jpg?width=300)
Auger electron spectroscopy (AES; pronounced [oʒe] in French) is a common analytical technique used specifically in the study of surfaces and, more generally, in the area of materials science. Underlying the spectroscopic technique is the Auger effect, as it has come to be called, which is based on the analysis of energetic electrons emitted from an excited atom after a series of internal relaxation events. The Auger effect was discovered independently by both Lise Meitner and Pierre Auger in the 1920s. Though the discovery was made by Meitner and initially reported in the journal Zeitschrift für Physik in 1922, Auger is credited with the discovery in most of the scientific community. Until the early 1950s Auger transitions were considered nuisance effects by spectroscopists, not containing much relevant material information, but studied so as to explain anomalies in x-ray spectroscopy data. Since 1953 however, AES has become a practical and straightforward characterization technique for probing chemical and compositional surface environments and has found applications in metallurgy, gas-phase chemistry, and throughout the microelectronics industry.