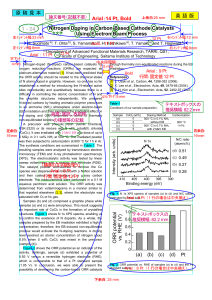
Chemical Reactions and Stoichiometry
... a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of a Chemical Reaction – the atoms in one or more reactant rearrange wh ...
... a. Evolution of heat and light (simultaneously) b. Production of a gas (bubbles, odor change) c. Formation of a precipitate (solid, cloudy) d. Color change (not introduced by an outside source such as dye or ink) Characteristics of a Chemical Reaction – the atoms in one or more reactant rearrange wh ...
PRACTICE FINAL EXAM CHEMISTRY 152 This
... The Ecell for a particular electrochemical reaction was found to be Ecell = −1.38 V. This value of Ecell means that this reaction is [a] spontaneous and ∆G° is negative. [c] in an equilibrium state and ∆G° is zero. [e] endothermic and ∆H° is positive. ...
... The Ecell for a particular electrochemical reaction was found to be Ecell = −1.38 V. This value of Ecell means that this reaction is [a] spontaneous and ∆G° is negative. [c] in an equilibrium state and ∆G° is zero. [e] endothermic and ∆H° is positive. ...
F Practice Test #2 Solutions
... 9. High concentrations of aqueous solutions of potassium sulfide and nickel(II) nitrate are mixed together. Which statement is correct? A) No precipitate forms. B) NiS will precipitate from solution. C) No reaction will occur. D) Both KNO3 and NiS precipitate from solution. E) KNO3 will precipitate ...
... 9. High concentrations of aqueous solutions of potassium sulfide and nickel(II) nitrate are mixed together. Which statement is correct? A) No precipitate forms. B) NiS will precipitate from solution. C) No reaction will occur. D) Both KNO3 and NiS precipitate from solution. E) KNO3 will precipitate ...
Measurements/Unit Cancellation/Significant Figures 1. When
... 43. Carbon monoxide can be combined with hydrogen to produce methanol, CH3OH. Methanol is used as an industrial solvent, as a reactant in synthesis, and as a clean-burning fuel for some racing cars. If you had 152.5 kg CO and 24.50 kg H2, how many kilograms of CH3OH could be produced? ...
... 43. Carbon monoxide can be combined with hydrogen to produce methanol, CH3OH. Methanol is used as an industrial solvent, as a reactant in synthesis, and as a clean-burning fuel for some racing cars. If you had 152.5 kg CO and 24.50 kg H2, how many kilograms of CH3OH could be produced? ...
Chemical reactions
... exothermic (use as a mnemonic the case of water, which formation reaction coincides with the combustion reaction H2+½O2=H2O and hf⊕=−286 kJ/mol); for alkanes, for instance, they are all negative with absolute values increasing with molecular size: −75 kJ/mol for CH4, −126 kJ/mol for n-C4H10, −250 kJ ...
... exothermic (use as a mnemonic the case of water, which formation reaction coincides with the combustion reaction H2+½O2=H2O and hf⊕=−286 kJ/mol); for alkanes, for instance, they are all negative with absolute values increasing with molecular size: −75 kJ/mol for CH4, −126 kJ/mol for n-C4H10, −250 kJ ...
Atoms, Molecules, and Ions
... assignments, and the veracity of signatures on attendance sheets and other verification of participation in class activities. The policy also prohibits students from submitting the same work in more than one class without receiving written authorization in advance from both instructors. Under the po ...
... assignments, and the veracity of signatures on attendance sheets and other verification of participation in class activities. The policy also prohibits students from submitting the same work in more than one class without receiving written authorization in advance from both instructors. Under the po ...
Chapter 2 Kinetics of Chemical Reactions - diss.fu
... The ion-molecule reactions show a particular behavior, since they present a very small or even no activation barrier (see section 2.2.1). It was experimentally found that ion-molecule reactions have a pronounced negative temperature dependence, deviating from an Arrhenius type dependence.40–43 This ...
... The ion-molecule reactions show a particular behavior, since they present a very small or even no activation barrier (see section 2.2.1). It was experimentally found that ion-molecule reactions have a pronounced negative temperature dependence, deviating from an Arrhenius type dependence.40–43 This ...
Effects of antioxidants for the degradation of flame
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
... 6 MGy in 0.1 vol% NH3 at 500 °C. The irradiated powder was then subjected to carbonization at 800 °C for 1 h in Ar. The synthesis conditions are summarized in Table 1. The resulting samples were analyzed by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS). The electr ...
- dragicaminic.info
... Push-pull alkenes, consisting of one or two electron-donating groups (EDG) at the terminus of the C=C bond and one or two electron-withdrawing groups (EWG) at the other terminus, have been widely studied on the account of their low rotational barrier around the C-C double bond. This is attributed to ...
... Push-pull alkenes, consisting of one or two electron-donating groups (EDG) at the terminus of the C=C bond and one or two electron-withdrawing groups (EWG) at the other terminus, have been widely studied on the account of their low rotational barrier around the C-C double bond. This is attributed to ...
Period 6
... • Some different types of isomer molecules are a branched chain. • In those 2 types of molecules have atoms that ...
... • Some different types of isomer molecules are a branched chain. • In those 2 types of molecules have atoms that ...
Chapter 4 - Aqueous Reactions
... When one substance is oxidized, another is reduced. An oxidation-reduction reaction occurs. Or a redox reaction occurs. Oxidation: loss of electrons (more positive) Reduction: gain of electrons (less positive) ...
... When one substance is oxidized, another is reduced. An oxidation-reduction reaction occurs. Or a redox reaction occurs. Oxidation: loss of electrons (more positive) Reduction: gain of electrons (less positive) ...
4 - Chemistry Biochemistry and Bio
... INTERMOLECULAR BONDS HYDROGEN BOND This far we have been discussing the bonds inside one molecule. However, bonds and electrostatic interactions can exist also between two or several molecules. Such bonds are called intermolecular bonds. The Physiologic most important kind of intermolecular bonds is ...
... INTERMOLECULAR BONDS HYDROGEN BOND This far we have been discussing the bonds inside one molecule. However, bonds and electrostatic interactions can exist also between two or several molecules. Such bonds are called intermolecular bonds. The Physiologic most important kind of intermolecular bonds is ...
Specific Reactions Quiz.wpd
... c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the presence of random carbon products prevents balancing e) energy is lost because it remains trapped within any remnant fuel bonds ...
... c) since energy is still tied up in carbon product bonds, energy is not released all at once d) the presence of random carbon products prevents balancing e) energy is lost because it remains trapped within any remnant fuel bonds ...
Your views are welcomed upon the theme of
... outer shell or an octet of electrons in the outer shell. Helium has the former, but not the latter. Argon has the latter, but not a full outer shell. Only the atom of neon has both.) Discrete atoms that do not have this type of outer shell structure are seldom found in nature: so single atoms of car ...
... outer shell or an octet of electrons in the outer shell. Helium has the former, but not the latter. Argon has the latter, but not a full outer shell. Only the atom of neon has both.) Discrete atoms that do not have this type of outer shell structure are seldom found in nature: so single atoms of car ...
Chapter 8 Chemical Equations and Reactions
... In a single-displacement reaction, also known as a replacement reaction, one element replaces a similar element in a compound. Many single-displacement reactions take place in aqueous solution. ...
... In a single-displacement reaction, also known as a replacement reaction, one element replaces a similar element in a compound. Many single-displacement reactions take place in aqueous solution. ...
Exam 3 - Canvas by Instructure
... E. None—when properly balanced, this half reaction does NOT include hydrogen. 16. Consider the balanced redox reaction below: 2 MnO4- + 6 H+ + 5 H2O2 → 2 Mn2+ + 8 H2O + 5 O2 If this reaction was properly balanced under BASIC conditions (with the smallest WHOLE number coefficients), how many water mo ...
... E. None—when properly balanced, this half reaction does NOT include hydrogen. 16. Consider the balanced redox reaction below: 2 MnO4- + 6 H+ + 5 H2O2 → 2 Mn2+ + 8 H2O + 5 O2 If this reaction was properly balanced under BASIC conditions (with the smallest WHOLE number coefficients), how many water mo ...
Unit 2 Chemical Reactions
... Acetylene gas is a fuel used in welding torches, and it combines with oxygen to produce a very hot flame. Because it is an organic compound, it contains carbon. When it burns in pure oxygen, it should produce carbon dioxide. This gas is the product of complete combustion. If there is not enough oxyg ...
... Acetylene gas is a fuel used in welding torches, and it combines with oxygen to produce a very hot flame. Because it is an organic compound, it contains carbon. When it burns in pure oxygen, it should produce carbon dioxide. This gas is the product of complete combustion. If there is not enough oxyg ...
Chapter 4: Chemical Reactions Elements can be characterized as
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
... For a binary compound AX, the oxidation number is the number of electrons gained or lost by an atom of the element when it forms the compound. It is sometimes referred to as the oxidation state. Oxidation numbers (Table 4-10) are used to track electron transfer in oxidation-reduction (redox) reactio ...
Soluble salts
... A _________________is as an analytical procedure of determining the concentration of one substance in solution by reacting it with a solution of another substance whose concentration is known, called a titrant (or standard solution). To carry out the process, we add the titrant, using a buret, to a ...
... A _________________is as an analytical procedure of determining the concentration of one substance in solution by reacting it with a solution of another substance whose concentration is known, called a titrant (or standard solution). To carry out the process, we add the titrant, using a buret, to a ...
CHEM MINI-COURSE SERIES M1.2___
... In this Learning Activity Packet (LAP), you will begin to study chemical reactions, a topic which could be considered the heart of chemistry. You will learn (1) why there is a need to balance chemical equations, (2) how to balance simple chemical equations, and (3) how to classify different types of ...
... In this Learning Activity Packet (LAP), you will begin to study chemical reactions, a topic which could be considered the heart of chemistry. You will learn (1) why there is a need to balance chemical equations, (2) how to balance simple chemical equations, and (3) how to classify different types of ...
Unit - III - E
... Steric attraction occurs when molecules have shapes or geometries that are optimized for interaction with one another. In these cases molecules will react with each other most often in specific arrangements. Chain crossing — A random coil can't change from one conformation to a closely related shap ...
... Steric attraction occurs when molecules have shapes or geometries that are optimized for interaction with one another. In these cases molecules will react with each other most often in specific arrangements. Chain crossing — A random coil can't change from one conformation to a closely related shap ...
urbano, mariajose
... Holism: The principle that a higher level of order cannot be meaningfully explained by examining component parts in isolation. • An organism is a living whole greater than the sum of its parts. • For example, a cell dismantled to its chemical ingredients is no longer a cell. 4. Describe seven emerge ...
... Holism: The principle that a higher level of order cannot be meaningfully explained by examining component parts in isolation. • An organism is a living whole greater than the sum of its parts. • For example, a cell dismantled to its chemical ingredients is no longer a cell. 4. Describe seven emerge ...