Test Booklet
... 10 According to this balanced chemical equation, what volume of C2 H2 is required to form 40.0 L of CO2 ? 2C2 H2 (g) + 5O2 (g) → 2H2 O (g) + 4CO2 (g) ...
... 10 According to this balanced chemical equation, what volume of C2 H2 is required to form 40.0 L of CO2 ? 2C2 H2 (g) + 5O2 (g) → 2H2 O (g) + 4CO2 (g) ...
Modeling the Rate of Heterogeneous Reactions
... Lateral interactions can be incorporated into this abstract view as well. From the modeling point of view one distinguishes between hard sphere and soft interactions. Hard sphere interactions are very strong lateral interactions, in which the adsorbed species behave as hard spheres and exclude neigh ...
... Lateral interactions can be incorporated into this abstract view as well. From the modeling point of view one distinguishes between hard sphere and soft interactions. Hard sphere interactions are very strong lateral interactions, in which the adsorbed species behave as hard spheres and exclude neigh ...
1 Chemical Reactions: Chemistry Word Equations • Write the names
... 2 H2O – H= H= – O= O= Balancing Rules 1. Determine the correct ____________________ for all the reactants and products. 2. Write the _______________________ equation. (Reactants on left, products on right, yield sign in between. If two or more reactants/products are involved, separate their formulas ...
... 2 H2O – H= H= – O= O= Balancing Rules 1. Determine the correct ____________________ for all the reactants and products. 2. Write the _______________________ equation. (Reactants on left, products on right, yield sign in between. If two or more reactants/products are involved, separate their formulas ...
chp0-Intro
... Warning: The textbook is inconsistent in denoting radicals. In many cases it shows a “dot” to indicate the one unpaired electron. However, some examples in the textbook do not have the dot so the reader is left to assume the species is a radical. You should know that species such as OH, CH3, ClO, H ...
... Warning: The textbook is inconsistent in denoting radicals. In many cases it shows a “dot” to indicate the one unpaired electron. However, some examples in the textbook do not have the dot so the reader is left to assume the species is a radical. You should know that species such as OH, CH3, ClO, H ...
Equilibrium (Sheet 1)
... CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an increase in the number of molecules possessing the activation energy, thus the rate of the forward ...
... CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an increase in the number of molecules possessing the activation energy, thus the rate of the forward ...
3.10 Neutralization
... • Net ionic equations for reactions between strong acids and bases HCl(aq) + KOH(aq) → KCl(aq) + H2O(l) H+ + Cl- + K+ + OH- → K+ + Cl- + H2O(l) ⇒H+ + OH- → H2O(l) – H+ is present in the form of H3O+ ...
... • Net ionic equations for reactions between strong acids and bases HCl(aq) + KOH(aq) → KCl(aq) + H2O(l) H+ + Cl- + K+ + OH- → K+ + Cl- + H2O(l) ⇒H+ + OH- → H2O(l) – H+ is present in the form of H3O+ ...
this PDF file
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
... reaction. However it is not easy to accurately calculate and plot the standard free energy changes and equilibrium constants for reactions due to the calculation complexity of reactions and phase transitions. It is found in the literature (Li, 2001) that it is not simple and convenient for calculati ...
Ionic bonding - Nidderdale High School
... increases number of collisions and increases rate Temperature: Particles have more energy and move faster and collide more often. More particles have energy greater than the activation energy so more successful collisions Catalyst: Catalysts change the rate of chemical reactions but are not used up ...
... increases number of collisions and increases rate Temperature: Particles have more energy and move faster and collide more often. More particles have energy greater than the activation energy so more successful collisions Catalyst: Catalysts change the rate of chemical reactions but are not used up ...
Chemical Reactions - hrsbstaff.ednet.ns.ca
... • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reactants. • Water is the product. ...
... • A chemical reaction is a chemical change where chemical substances (called reactants) react to give new chemical substances (called products). • Example – The combustion of hydrogen in oxygen is a chemical reaction which gives water. • Hydrogen and Oxygen are the reactants. • Water is the product. ...
Theoretical Competition - Austrian Chemistry Olympiad
... The compound G is also white and poorly soluble in water, but more readily soluble than the other homologous compounds of this group. It is also generated by the reaction of the element with water. ...
... The compound G is also white and poorly soluble in water, but more readily soluble than the other homologous compounds of this group. It is also generated by the reaction of the element with water. ...
New AQA C3 revison guide
... The periodic tables patterns are now known to be based on the structure of the atom. Elements in the periodic table are arranged in order of atomic number (number of protons in the nucleus) The group number of an element shows the number of electrons in the outer shell. The period number shows the n ...
... The periodic tables patterns are now known to be based on the structure of the atom. Elements in the periodic table are arranged in order of atomic number (number of protons in the nucleus) The group number of an element shows the number of electrons in the outer shell. The period number shows the n ...
2.5 THE NAMES AND FORMULAS OF COMPOUNDS
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
... explain many of the properties of ionic compounds, but they aren’t sufficient to explain the physical state of molecular compounds. If covalent bonds were the only forces at work, molecular compounds would all be gases, as there would be no attraction between the molecules strong enough to order the ...
Stoichiometry
... Two compounds are involved with the cation of one compound EXCHANGING with the cation of another compound. AX + BZ AZ + BX These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The ...
... Two compounds are involved with the cation of one compound EXCHANGING with the cation of another compound. AX + BZ AZ + BX These reactions proceed if one of the ff. is satisfied: 1. An insoluble/slightly soluble product is formed (PRECIPITATE formation) 2. A weakly ionized species is produced. The ...
ch14
... Compounds of 3A elements have more covalent character than similar 2A compounds. Aluminum has the physical properties of a metal, but its halides exist as covalent dimers. ...
... Compounds of 3A elements have more covalent character than similar 2A compounds. Aluminum has the physical properties of a metal, but its halides exist as covalent dimers. ...
Chemistry - Edexcel
... 1 Some of the gases used in industry are stored in cylinders. (a) The cylinders are painted in different colours according to which gas is stored in them. Why is it an advantage to use different colours? ...
... 1 Some of the gases used in industry are stored in cylinders. (a) The cylinders are painted in different colours according to which gas is stored in them. Why is it an advantage to use different colours? ...
Re-typed from The Ultimate Chemical Equations Handbook by
... of water molecules needs to be adjusted. 5. If there is an odd number of an element on one side and an even number on the other, the odd number will need to be evened out – so use a coefficient of 2 for that substance. 6. If there are polyatomic ions that remain together as a unit during the reactio ...
... of water molecules needs to be adjusted. 5. If there is an odd number of an element on one side and an even number on the other, the odd number will need to be evened out – so use a coefficient of 2 for that substance. 6. If there are polyatomic ions that remain together as a unit during the reactio ...
Energy of Reactions
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
... The reason why the entropy of this reaction decreased is that you are going from 4 moles of gas to 2 moles of gas. The amount of gas is reduced and lowers the entropy. δGrxn = -33.1kJ Since the Gibb’s free energy is negative, this is a spontaneous reaction. ...
11-1 SECTION 11 THERMOCHEMISTRY Thermochemistry: Study of
... implies that when 2 moles of gaseous dihydrogen reacts with one mole of gaseous dioxygen to give two moles of liquid water 570 kilojoules of energy is released from the reacting system to its surroundings.] The ∆ means change, r stands for reaction and H is the symbol for enthalpy. ∆rH is the enthal ...
... implies that when 2 moles of gaseous dihydrogen reacts with one mole of gaseous dioxygen to give two moles of liquid water 570 kilojoules of energy is released from the reacting system to its surroundings.] The ∆ means change, r stands for reaction and H is the symbol for enthalpy. ∆rH is the enthal ...
Ch 4 Types of Chemical Reactions and Solution Stoichiometry
... Hydrogen is almost always +1; metal hydrides are an exception, where it is -1 (in these situations, hydrogen is placed at the end of a chemical formula like LiH) The sum of the oxidation states must be zero for a neutral compound; for polyatomic ions, the sum of the oxidation states must equal t ...
... Hydrogen is almost always +1; metal hydrides are an exception, where it is -1 (in these situations, hydrogen is placed at the end of a chemical formula like LiH) The sum of the oxidation states must be zero for a neutral compound; for polyatomic ions, the sum of the oxidation states must equal t ...
Preparation of Supported Catalysts
... 2. At least one additional layer near to the surface with properties different from the bulk solvent. → melting and boiling point and dielectric constant different from bulk water; → unmixing / separation in water containing organic solvents → formation of a thin aqueous layer at the solid surface ( ...
... 2. At least one additional layer near to the surface with properties different from the bulk solvent. → melting and boiling point and dielectric constant different from bulk water; → unmixing / separation in water containing organic solvents → formation of a thin aqueous layer at the solid surface ( ...
C4C5C6
... number (same number of protons) but a different number of neutrons so it has a different relative ...
... number (same number of protons) but a different number of neutrons so it has a different relative ...
1 R R 1Ch Ro_ R___ + ____ ____ + _+ S ___y → +
... Use this completion exercise to check your understanding of the concepts and terms that are introduced in this section. Each blank can be completed with a term, short phrase, or number. ...
... Use this completion exercise to check your understanding of the concepts and terms that are introduced in this section. Each blank can be completed with a term, short phrase, or number. ...
Introduction to Organic Chemistry Curriculum
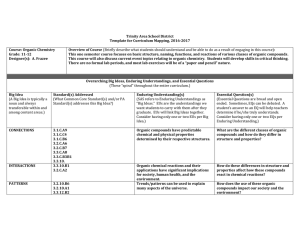
... Overview of Course (Briefly describe what students should understand and be able to do as a result of engaging in this course): This one semester course focuses on basic structure, naming, functions, and reactions of various classes of organic compounds. This course will also discuss current event t ...
... Overview of Course (Briefly describe what students should understand and be able to do as a result of engaging in this course): This one semester course focuses on basic structure, naming, functions, and reactions of various classes of organic compounds. This course will also discuss current event t ...