
Chapter 1: Fundamental Concepts
... Polarity of Molecules • Can predict from molecular shape • Polar or Non-Polar? – In very symmetrical structures (e.g. CO2 or CF4), the individual bond dipoles effectively cancel each other and the molecule is ...
... Polarity of Molecules • Can predict from molecular shape • Polar or Non-Polar? – In very symmetrical structures (e.g. CO2 or CF4), the individual bond dipoles effectively cancel each other and the molecule is ...
CHAPtER 9 Properties and reactions of organic compounds
... The millions of organic compounds known to chemists are mainly covalent molecules. These molecules could be simple molecules, such as methane and ethanol, or macromolecules, such as polyethene and proteins. The physical properties and behaviours of organic molecules are influenced by their size, sha ...
... The millions of organic compounds known to chemists are mainly covalent molecules. These molecules could be simple molecules, such as methane and ethanol, or macromolecules, such as polyethene and proteins. The physical properties and behaviours of organic molecules are influenced by their size, sha ...
IB:Enthalpy Review Questions
... a. Derive an expression that can be used to find ∆Hrxn values found from the bond energies involved. b. Discuss the fact that ∆Hrxn values found from bond energies often differ from those calculated using ...
... a. Derive an expression that can be used to find ∆Hrxn values found from the bond energies involved. b. Discuss the fact that ∆Hrxn values found from bond energies often differ from those calculated using ...
3.0 Hess`s Law
... knowing anything else about how the reaction occurs. • Mathematically, the overall equation for enthalpy change will be in the form of the following equation: ...
... knowing anything else about how the reaction occurs. • Mathematically, the overall equation for enthalpy change will be in the form of the following equation: ...
Aqueous Solutions
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
chapter 5 - chemical reactions
... Some double-displacement reactions may result in both the formation of precipitates and water. They can be classified as both precipitation and acid-base reactions. For examples: 1. Ba(OH)2(aq) + H2SO4(aq) BaSO4(s) + 2 H2O(l) 2. 3Ca(OH)2(aq) + 2H3PO4(aq) Ca3(PO4)2(s) + 6 H2O(l) Ionic Equations f ...
... Some double-displacement reactions may result in both the formation of precipitates and water. They can be classified as both precipitation and acid-base reactions. For examples: 1. Ba(OH)2(aq) + H2SO4(aq) BaSO4(s) + 2 H2O(l) 2. 3Ca(OH)2(aq) + 2H3PO4(aq) Ca3(PO4)2(s) + 6 H2O(l) Ionic Equations f ...
An Efficient Oxidation of Benzoins to Benzils by Manganese (II
... activity and selectivity, the technical problems encountered ...
... activity and selectivity, the technical problems encountered ...
FREE Sample Here
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
... 1. List several differences between ionic and covalent bonds. Ionic bonds occur when ions of opposite charge are mutually attracted. Acids and bases are examples of ionic compounds. Covalent bonds are strong chemical bonds that occur when atoms share electrons. Methane and sugar are examples of cova ...
Problems - Department of Chemistry HKU
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
PRE AP CHEMISTRY REVIEW PROBLEMS NON COLLEGE
... i. What is the final volume of a 0.600 M KNO3 solution prepared with 50.0 mL of a 1.50 M solution? j. What is the concentration of a stock solution of HC2H3O2 from which 20.0 mL was used to prepare a 200 mL sample of a 0.050 M solution? ...
... i. What is the final volume of a 0.600 M KNO3 solution prepared with 50.0 mL of a 1.50 M solution? j. What is the concentration of a stock solution of HC2H3O2 from which 20.0 mL was used to prepare a 200 mL sample of a 0.050 M solution? ...
Document
... o average energy (dashed line) of substrates (higher on graph) and products (lower on graph) o delta G starts at substrate line and goes to transition state o adding a catalyst lowers the free energy of the transition state o In an uncatalyzed reaction, you must go through some sort of transition st ...
... o average energy (dashed line) of substrates (higher on graph) and products (lower on graph) o delta G starts at substrate line and goes to transition state o adding a catalyst lowers the free energy of the transition state o In an uncatalyzed reaction, you must go through some sort of transition st ...
CHEM1001 2012-J-2 June 2012 22/01(a) • Complete the following
... dispersion and dipole-dipole forces in the other compounds and hence these two compounds have the highest boiling points. CH3CH2OH has more dispersion forces than CH3OH, so it has the highest boiling point. • Melting points of the hydrogen halides increase in the order HCl < HBr < HF < HI. ...
... dispersion and dipole-dipole forces in the other compounds and hence these two compounds have the highest boiling points. CH3CH2OH has more dispersion forces than CH3OH, so it has the highest boiling point. • Melting points of the hydrogen halides increase in the order HCl < HBr < HF < HI. ...
Thermochemistry - Ars
... The enthalpy of a reaction from the thermochemical equation is a conversion factor that can be used to calculate the amount of heat generated or absorbed by a reaction for an amount different than the stoichiometric amounts from the balanced chemical equation. The conversion factors are based on the ...
... The enthalpy of a reaction from the thermochemical equation is a conversion factor that can be used to calculate the amount of heat generated or absorbed by a reaction for an amount different than the stoichiometric amounts from the balanced chemical equation. The conversion factors are based on the ...
Chapter 4: Solution Chemistry and the Hydrosphere
... ___ Mg(NO3)2(aq) + ___ K3PO4(aq) ___ Mg3(PO4)2(s) + ___ KNO3(aq) ___ Ni(OH)3(s) + ___ HCl(aq) ___ H2O(l) + ___ NiCl3(aq) ___ Al(HCO3)3(aq) Δ ___ CO2(g) + ___ H2O(g) + ___ Al2(CO3)3(s) ___ Fe(s) + ___ Pb(NO3)2(aq) ___ Pb(s) + ___ Fe(NO3)3(aq) ...
... ___ Mg(NO3)2(aq) + ___ K3PO4(aq) ___ Mg3(PO4)2(s) + ___ KNO3(aq) ___ Ni(OH)3(s) + ___ HCl(aq) ___ H2O(l) + ___ NiCl3(aq) ___ Al(HCO3)3(aq) Δ ___ CO2(g) + ___ H2O(g) + ___ Al2(CO3)3(s) ___ Fe(s) + ___ Pb(NO3)2(aq) ___ Pb(s) + ___ Fe(NO3)3(aq) ...
Chapter 4 - profpaz.com
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
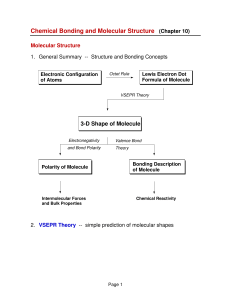
3-D Shape of Molecule
... double bond -- combination of one σ bond and one π bond triple bond -- ...
... double bond -- combination of one σ bond and one π bond triple bond -- ...
Chemistry of the Non
... We divide the periodic table into metals, nonmetals and metalloids. Nonmetals occupy the upper right portion of the periodic table. • H is a special case. Electronegativity is important when determining whether an element is a metal. Nonmetals tend to have higher electronegativities than metals. • T ...
... We divide the periodic table into metals, nonmetals and metalloids. Nonmetals occupy the upper right portion of the periodic table. • H is a special case. Electronegativity is important when determining whether an element is a metal. Nonmetals tend to have higher electronegativities than metals. • T ...
127 - Chimica
... The series of reactions shown in Scheme I were then proven. In this way, all the known dinuclear hydridocarbonyl complexes of rheniumg are chemically related. The structure of the [Re,H,(p-H)(CO),]- anion is shown in Figure 1. It possesses an idealized C2 symmetry and a staggered (torsion angle C13- ...
... The series of reactions shown in Scheme I were then proven. In this way, all the known dinuclear hydridocarbonyl complexes of rheniumg are chemically related. The structure of the [Re,H,(p-H)(CO),]- anion is shown in Figure 1. It possesses an idealized C2 symmetry and a staggered (torsion angle C13- ...
Factors that affect the rate of reactions
... MOVE FASTER, HIT each other MORE OFTEN with MORE ENERGY The reaction rate will INCREASE ...
... MOVE FASTER, HIT each other MORE OFTEN with MORE ENERGY The reaction rate will INCREASE ...
Summer Work
... First Exercise: Fe3+(aq) + SCN-(aq) FeSCN2+(aq) If at equilibrium and 25°C, you have [FeSCN2+] = 0.25 mol/L, [Fe3+] = 0.046 mol/L, and [SCN-] = 0.046 mol/L, what is the equilibrium constant, Keq? ...
... First Exercise: Fe3+(aq) + SCN-(aq) FeSCN2+(aq) If at equilibrium and 25°C, you have [FeSCN2+] = 0.25 mol/L, [Fe3+] = 0.046 mol/L, and [SCN-] = 0.046 mol/L, what is the equilibrium constant, Keq? ...
Oxidation And Degradation Products Of Common Oxygen Scavengers
... protective oxide films on the metal surfaces of the steam/water system. Thus, the reaction with metal oxides is equally as important as the reaction with dissolved oxygen. During the past decade, a number of materials have been proposed for use as oxygen scavengers and metal passivators. The reactio ...
... protective oxide films on the metal surfaces of the steam/water system. Thus, the reaction with metal oxides is equally as important as the reaction with dissolved oxygen. During the past decade, a number of materials have been proposed for use as oxygen scavengers and metal passivators. The reactio ...















![C2_revision_slides_V3_+_questions_+_MS_-_H[1]](http://s1.studyres.com/store/data/000092833_1-97fb33725e7f1ef12029ed42751d3dca-300x300.png)






