Key - GCC
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
... 1. List the three general classes of chemical reactions: precipitation, acid-base neutralization, and redox reactions 2. How can you identify each of the three reaction types above (e.g., what characteristic defines each one?)? Precipitation reactions have solid products, also all reactants and prod ...
Chapter 4: Aqueous Reactions and Solution
... You will be expected to be able to write complete (molecular) and net ionic chemical equations. This will require you to be disciplined, committing yourself to memorizing certain information and doing much practice. To be successful you must develop the ability to recognize an acid, a base and an io ...
... You will be expected to be able to write complete (molecular) and net ionic chemical equations. This will require you to be disciplined, committing yourself to memorizing certain information and doing much practice. To be successful you must develop the ability to recognize an acid, a base and an io ...
Document
... Check for Understanding Aqueous potassium nitrate and a precipitate of barium chromate are formed when aqueous solutions of barium nitrate and potassium chromate are mixed. Ba(NO3)2 (aq) + K2CrO4 (aq) ...
... Check for Understanding Aqueous potassium nitrate and a precipitate of barium chromate are formed when aqueous solutions of barium nitrate and potassium chromate are mixed. Ba(NO3)2 (aq) + K2CrO4 (aq) ...
Class XI Chemistry Practics Paper
... Q3 Which property of element is used to classify them in long form of periodic table? Q4 Write resonance structure of Ozone or sulphurdioxide. Q5 Write conjugate base for water and NH4+ species. Q6 What do you understand by Hydrogen economy? Q7 Find out oxidation number of chromium in K2Cr2O7 molecu ...
... Q3 Which property of element is used to classify them in long form of periodic table? Q4 Write resonance structure of Ozone or sulphurdioxide. Q5 Write conjugate base for water and NH4+ species. Q6 What do you understand by Hydrogen economy? Q7 Find out oxidation number of chromium in K2Cr2O7 molecu ...
Question paper - Unit A173/02 - Module C7 - Higher tier (PDF
... The sugar is obtained from crops such as sugar beet or sugar cane. The sugar is fermented with yeast at a temperature of about 30 °C. (a) The sustainability of chemical processes depends on a number of factors. One of these factors is the renewability of raw materials. Consider this, and other facto ...
... The sugar is obtained from crops such as sugar beet or sugar cane. The sugar is fermented with yeast at a temperature of about 30 °C. (a) The sustainability of chemical processes depends on a number of factors. One of these factors is the renewability of raw materials. Consider this, and other facto ...
Question paper - Edexcel
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with a If you change your mind, put a line through the box cross . 1 In which of the ...
... Answer ALL the questions in this section. You should aim to spend no more than 20 minutes on this section. For each question, select one answer from A to D and put a cross in the box . and then mark your new answer with a If you change your mind, put a line through the box cross . 1 In which of the ...
+ H 2 O(l)
... AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Blue color for the products represents the driving force which allows the chemical reaction to occur. ...
... AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Blue color for the products represents the driving force which allows the chemical reaction to occur. ...
problems - chem.msu.su
... 3. Determine the acid НА (HCl, HClO4 or HBr) and alkali (LiOH, KOH or NaOH), taking into account that the molar conductivities of ions [mS·m2/mol] are as follows: ...
... 3. Determine the acid НА (HCl, HClO4 or HBr) and alkali (LiOH, KOH or NaOH), taking into account that the molar conductivities of ions [mS·m2/mol] are as follows: ...
IGCSE Revision Guide (Double Award) | PDF
... understand and explain how atoms form ions and that metals and hydrogen form positive ions and non-‐ metals form negative ions ...
... understand and explain how atoms form ions and that metals and hydrogen form positive ions and non-‐ metals form negative ions ...
CHEMICAL BONDING
... • A few atoms seem to be stable being “electron deficient” • The atoms are Be, B, Mg and Al • So we can have molecules like BF3 ...
... • A few atoms seem to be stable being “electron deficient” • The atoms are Be, B, Mg and Al • So we can have molecules like BF3 ...
- Department of Chemistry, York University
... NB: C4H- would be very interesting because C4H is massively abundant in IRC+10216. The cyanopolyynyl radicals like C5N are also very promising because they have EA values of 4 eV or more, so attachment is very favourable, but these radicals aren't as abundant as ...
... NB: C4H- would be very interesting because C4H is massively abundant in IRC+10216. The cyanopolyynyl radicals like C5N are also very promising because they have EA values of 4 eV or more, so attachment is very favourable, but these radicals aren't as abundant as ...
Today Electrochemistry electrons moving about equilibrium with a
... add half reactions together to balance electrons multiply each half reaction by proper factor to get the same number of electron in each reaction to convert to reaction in base neutralize H+ with OH- eliminate any H+, OH-, or H2O that appears on both sides of the equation ...
... add half reactions together to balance electrons multiply each half reaction by proper factor to get the same number of electron in each reaction to convert to reaction in base neutralize H+ with OH- eliminate any H+, OH-, or H2O that appears on both sides of the equation ...
Today Electrochemistry electrons moving about equilibrium with a
... In a compound with no metals H is assign to +1! H2O H is 1+! HCl H is 1+! note: H2 is not a compound! ...
... In a compound with no metals H is assign to +1! H2O H is 1+! HCl H is 1+! note: H2 is not a compound! ...
Exam Review Chapter 18-Equilibrium
... c. neither a nor b b. chemical equilibrium. d. both a and b 8. According to collision theory, in order for a chemical reaction to occur, the reactant atoms must: a. make contact with each other. b. have a minimum level of kinetic energy. c. form an activated complex. d. all of the above ...
... c. neither a nor b b. chemical equilibrium. d. both a and b 8. According to collision theory, in order for a chemical reaction to occur, the reactant atoms must: a. make contact with each other. b. have a minimum level of kinetic energy. c. form an activated complex. d. all of the above ...
Practice Final Exam, Chemistry 2220, Organic Chem II 1. Rank the
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
... 6. Which one of the following compounds is NOT a product of reaction between 1,3butadiene and HBr? A. (S)-3-bromo-1-butene B. (R)-3-bromo-1-butene C. (Z)-2-bromo-2-butene D. (E)-1-bromo-2-butene 7. Choose the reagents necessary to carry out the following conversion. O ...
Final competitions (29.03.2008) Competing equilibria Complex
... Many compounds used as medicines, for example ephedrine and hydroxyzine, contain aromatic rings. Often, convenient substrate for their synthesis is benzaldehyde and its derivatives. A. Ephedrine, a natural product with molecular weight of 165.2 g mol-1, has been used for thousands of years in tradit ...
... Many compounds used as medicines, for example ephedrine and hydroxyzine, contain aromatic rings. Often, convenient substrate for their synthesis is benzaldehyde and its derivatives. A. Ephedrine, a natural product with molecular weight of 165.2 g mol-1, has been used for thousands of years in tradit ...
Writing Chemical Formulas and Chemical Reactions
... place. Arsenic’s name remains unchanged when the higher oxidation state is used. For some elements, the last syllable is not removed (i.e., Co, Ni). The suffix "ous" indicates the lower oxidation state was used for the cation. The suffix "ic" indicates the higher oxidation state was used for the cat ...
... place. Arsenic’s name remains unchanged when the higher oxidation state is used. For some elements, the last syllable is not removed (i.e., Co, Ni). The suffix "ous" indicates the lower oxidation state was used for the cation. The suffix "ic" indicates the higher oxidation state was used for the cat ...
Support material for lesson planning – AS content
... Module 1.1 (Practical skills assessed in a written examination) is not included explicitly in the table below. The expectation is that practical skills are developed through the practical work done throughout the course and in support of conceptual understanding. Suggestions for suitable practical w ...
... Module 1.1 (Practical skills assessed in a written examination) is not included explicitly in the table below. The expectation is that practical skills are developed through the practical work done throughout the course and in support of conceptual understanding. Suggestions for suitable practical w ...
Unit 4, Lesson #3 - Patterson Science
... solutions to calculate the rate of a reaction, measuring these values can also be used to calculate the concentrations of the different species. Once the concentrations have been determined, these values are “plugged into” the expression and Keq is calculated. The wonderful thing about Keq is that i ...
... solutions to calculate the rate of a reaction, measuring these values can also be used to calculate the concentrations of the different species. Once the concentrations have been determined, these values are “plugged into” the expression and Keq is calculated. The wonderful thing about Keq is that i ...
KIN1PP - Knockhardy
... • Powdered solids react quicker than larger lumps • Catalysts (e.g. in catalytic converters) are finely divided for this reason ...
... • Powdered solids react quicker than larger lumps • Catalysts (e.g. in catalytic converters) are finely divided for this reason ...
AP Chemistry - Chagrin Falls Schools
... Major Projects: 5% each day; after five days, no credit will be given Everyday homework: 50% credit for a day late; after one day, no credit will be given Major Projects: 10% each day; after three days, no credit will be given Everyday homework: 50% for one day late; after the first day late, no cre ...
... Major Projects: 5% each day; after five days, no credit will be given Everyday homework: 50% credit for a day late; after one day, no credit will be given Major Projects: 10% each day; after three days, no credit will be given Everyday homework: 50% for one day late; after the first day late, no cre ...
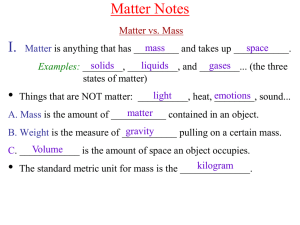
Chem A Week 2 Matter Notes
... of superheated “soup” of bits and pieces of atoms. It only exists ...
... of superheated “soup” of bits and pieces of atoms. It only exists ...
Topic2890 Thermodynamics and Kinetics A given system at
... which chemists understand in terms of spontaneous chemical reaction (in a closed system). The task for chemists is to identify the actual chemical reaction in a given closed system. Thermodynamics and Chemical Kinetics ...
... which chemists understand in terms of spontaneous chemical reaction (in a closed system). The task for chemists is to identify the actual chemical reaction in a given closed system. Thermodynamics and Chemical Kinetics ...