Chemical Equilibrium - Department of Chemistry
... Q: Will the amount of NO2(g) formed be greater at high or low temperature? A: The forward reaction as written is endothermic. An increase in temperature will favour a shift to the right in equilibrium. NO2(g) will be higher at higher temperature. ...
... Q: Will the amount of NO2(g) formed be greater at high or low temperature? A: The forward reaction as written is endothermic. An increase in temperature will favour a shift to the right in equilibrium. NO2(g) will be higher at higher temperature. ...
visual problems - Western Oregon University
... 12.61. Use the data in Appendix 4 to calculate ∆H° and ∆S° for the vaporization of hydrogen peroxide: H2O2(ℓ) → H2O2(g) Assuming that the calculated values are independent of temperature, what is the boiling point of hydrogen peroxide at P = 1.00 atm? 12.62. Volcanoes Deposits of elemental sulfur ar ...
... 12.61. Use the data in Appendix 4 to calculate ∆H° and ∆S° for the vaporization of hydrogen peroxide: H2O2(ℓ) → H2O2(g) Assuming that the calculated values are independent of temperature, what is the boiling point of hydrogen peroxide at P = 1.00 atm? 12.62. Volcanoes Deposits of elemental sulfur ar ...
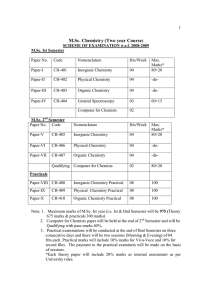
M.Sc. Chemistry (Two year Course)
... reactions). General treatment of chain reactions (ortho -para hydrogen conversion and hydrogen - bromine reactions), apparent activation energy of chain reactions, chain length, Rice- Herzfeld mechanism of organic molecules decomposition(acetaldehyde) Branching chain reactions and explosions ( H2 - ...
... reactions). General treatment of chain reactions (ortho -para hydrogen conversion and hydrogen - bromine reactions), apparent activation energy of chain reactions, chain length, Rice- Herzfeld mechanism of organic molecules decomposition(acetaldehyde) Branching chain reactions and explosions ( H2 - ...
Chapter 15. Chemical Equilibrium
... • The constants are defined in terms of activities rather than concentrations or partial pressures. • The activity of a substance in an ideal mixture is the ratio of the concentration or pressure of the substance either to a reference concentration (1 M) or a reference pressure (1 atm). • The units ...
... • The constants are defined in terms of activities rather than concentrations or partial pressures. • The activity of a substance in an ideal mixture is the ratio of the concentration or pressure of the substance either to a reference concentration (1 M) or a reference pressure (1 atm). • The units ...
Question Bank - Edudel.nic.in
... this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3 use this information to calcula ...
... this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3 use this information to calcula ...
Chemistry Review 2 answer key
... 'see explanation below' 24. Base your answer on the information below. Aluminum is one of the most abundant metals in Earth's crust. The aluminum compound found in bauxite ore is Al2O3. Over one hundred years ago, it was difficult and expensive to isolate aluminum from bauxite ore. In 1886, a brothe ...
... 'see explanation below' 24. Base your answer on the information below. Aluminum is one of the most abundant metals in Earth's crust. The aluminum compound found in bauxite ore is Al2O3. Over one hundred years ago, it was difficult and expensive to isolate aluminum from bauxite ore. In 1886, a brothe ...
Chemical Equilibrium - Request a Spot account
... the heat of the system; however, because heat is not part of the Keq equation, the Keq value will change to reflect the new concentrations of the reactants and products. (Before reading the next section on temperature changes, remember, heat and temperature are not the same thing. A substance at its ...
... the heat of the system; however, because heat is not part of the Keq equation, the Keq value will change to reflect the new concentrations of the reactants and products. (Before reading the next section on temperature changes, remember, heat and temperature are not the same thing. A substance at its ...
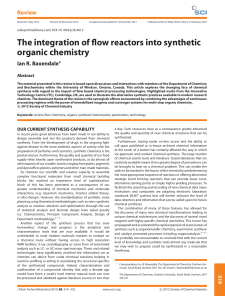
The integration of flow reactors into synthetic organic chemistry
... involve only one bond-forming or bond-breaking event, a series of up to six additional manipulations (work-up and purification) can be required. Interestingly, these supplementary operations, which are essential but costly (in time and resources), add very little intrinsic value to the compounds; th ...
... involve only one bond-forming or bond-breaking event, a series of up to six additional manipulations (work-up and purification) can be required. Interestingly, these supplementary operations, which are essential but costly (in time and resources), add very little intrinsic value to the compounds; th ...
chemistry
... prediction. If you read a description of matter which indicates that it is a solid, nonmetallic molecular compound, then (by the end of this textbook at least) you will have a good idea of its properties in general. There are many different classification systems used, some linked together, others s ...
... prediction. If you read a description of matter which indicates that it is a solid, nonmetallic molecular compound, then (by the end of this textbook at least) you will have a good idea of its properties in general. There are many different classification systems used, some linked together, others s ...
Reaction Rates/Chemical Kinetics
... – Lower temperature, slower reaction rate – Higher temperatures make molecules move faster because they have more kinetic energy so reaction is more likely ...
... – Lower temperature, slower reaction rate – Higher temperatures make molecules move faster because they have more kinetic energy so reaction is more likely ...
oxidation–reduction reaction
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
... • A reaction in which electrons are transferred from one atom to another is called an oxidation–reduction reaction. • Also called redox reactions ...
Influence of Hydrogen Atoms on the Growth of carbon based
... sp2 and hydrogen content in the form of a ternary phase diagram (see Fig. 1.2) as first was done by Smith [3] and later used by Jacob and Möller [4]. In the sp3 corner are diamond-like films with a significant fraction of sp3 bonds. In the lower left corner (sp2 ) are films with graphitic structure ...
... sp2 and hydrogen content in the form of a ternary phase diagram (see Fig. 1.2) as first was done by Smith [3] and later used by Jacob and Möller [4]. In the sp3 corner are diamond-like films with a significant fraction of sp3 bonds. In the lower left corner (sp2 ) are films with graphitic structure ...
Chemical Reactions and Solution Stoichiometry
... Chemical reactions involve the transformation of matter, the reactants, into different materials, the products. A combination reaction is one in which typically two or more reactants, usually elements or compounds, combine to form one product, usually a compound. In one type of combination reaction, ...
... Chemical reactions involve the transformation of matter, the reactants, into different materials, the products. A combination reaction is one in which typically two or more reactants, usually elements or compounds, combine to form one product, usually a compound. In one type of combination reaction, ...
Lipid Hydroperoxide Activation of N-Hydroxy-N
... the arylamine carcinogens to the ultimate reactive chemical form are still an open question. However, much has been learned in recent years (see Refs. 15, 16, and 21 for re views). For example, it was discovered in 1960 (5) that the N-hydroxylation of AAF3 into N-OH-AAF considerably en hanced the po ...
... the arylamine carcinogens to the ultimate reactive chemical form are still an open question. However, much has been learned in recent years (see Refs. 15, 16, and 21 for re views). For example, it was discovered in 1960 (5) that the N-hydroxylation of AAF3 into N-OH-AAF considerably en hanced the po ...
PDF File
... of both substrate and potential ribozyme ligands can determine whether such groups are indeed ligands for particular metal ions (5, 17, 27). Possible complications that may arise in these analyses, but that do not apply to the Tetrahymena ribozyme, are described in the Supporting Information. Consid ...
... of both substrate and potential ribozyme ligands can determine whether such groups are indeed ligands for particular metal ions (5, 17, 27). Possible complications that may arise in these analyses, but that do not apply to the Tetrahymena ribozyme, are described in the Supporting Information. Consid ...
Pirogov National Medical Univercity of Vinnitsa
... Early beginning of each semester the teacher must instruct a group of students on policy compliance rules work in the chemical laboratory and safety equipment. Students must confirm the knowledge of safety rules in their own signatures in a magazine. Accidents (burns, wounds, poisoning) in the labor ...
... Early beginning of each semester the teacher must instruct a group of students on policy compliance rules work in the chemical laboratory and safety equipment. Students must confirm the knowledge of safety rules in their own signatures in a magazine. Accidents (burns, wounds, poisoning) in the labor ...
Lessons 9
... Specific Heat Capacity The specific heat capacity is the amount of heat energy that is needed to raise the temperature of one gram of a substance 1oC (or 1 K). Specific heat capacities vary from substance to substance, and even for different states (solid, liquid, gas) of the same substance. ...
... Specific Heat Capacity The specific heat capacity is the amount of heat energy that is needed to raise the temperature of one gram of a substance 1oC (or 1 K). Specific heat capacities vary from substance to substance, and even for different states (solid, liquid, gas) of the same substance. ...
K eq
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
... No - The paper wads keep flying in both directions even after equilibrium is achieved ...
Industrial Chemistry - Osun State University
... Atomic and nuclear Basis: Evidence for atomic constituents. Electrons, protons and neutrons – their relative charges and masses. The nucleus, atomic number, mass isotopes and mass spectra. The electronic structure of the atom. Radioactivity; X-ray radiation and detection. Nuclear transformation and ...
... Atomic and nuclear Basis: Evidence for atomic constituents. Electrons, protons and neutrons – their relative charges and masses. The nucleus, atomic number, mass isotopes and mass spectra. The electronic structure of the atom. Radioactivity; X-ray radiation and detection. Nuclear transformation and ...
Molecular-level mechanisms of quartz dissolution under neutral and
... the Si–O–Si bonding, which makes bond hydrolysis easier and accelerates the dissolution of the silicate mineral. Wallace et al. (2010) used first-principles quantum chemistry methods to study the hydrolysis of Si–O–Si linkages in Mg2+- and Ca2+-containing solutions or in pure water under near-neutra ...
... the Si–O–Si bonding, which makes bond hydrolysis easier and accelerates the dissolution of the silicate mineral. Wallace et al. (2010) used first-principles quantum chemistry methods to study the hydrolysis of Si–O–Si linkages in Mg2+- and Ca2+-containing solutions or in pure water under near-neutra ...
Chemistry – 5071
... on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need of students to develop skills that will be of long term value in an increasing technological world rather than focusing on large quantities of actual material which m ...
... on the understanding and application of scientific concepts and principles. This approach has been adopted in recognition of the need of students to develop skills that will be of long term value in an increasing technological world rather than focusing on large quantities of actual material which m ...
Chemical Equilibria - Beck-Shop
... At the start of the reaction, with just A and B present, only the forward reaction will occur. The rate of the forward reaction (determined by the gradient of the tangent drawn to the concentration versus time plot) is at its peak since [reactants] is at its highest while the rate of the backward re ...
... At the start of the reaction, with just A and B present, only the forward reaction will occur. The rate of the forward reaction (determined by the gradient of the tangent drawn to the concentration versus time plot) is at its peak since [reactants] is at its highest while the rate of the backward re ...