chemical bonding and molecular structure
... independent atom in nature, except noble gases. However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a molecule. Obviously there must be some force which holds these constituent atoms together in the molecules. The attr ...
... independent atom in nature, except noble gases. However, a group of atoms is found to exist together as one species having characteristic properties. Such a group of atoms is called a molecule. Obviously there must be some force which holds these constituent atoms together in the molecules. The attr ...
1411FINALSAMPLEs and Key
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
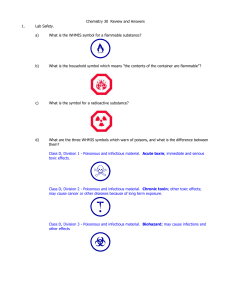
REVIEW and answers
... being malleable and ductile. Metals can also conduct electricity and heat. The more delocalized the electrons the greater these properties and the lower the boiling point and melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, m ...
... being malleable and ductile. Metals can also conduct electricity and heat. The more delocalized the electrons the greater these properties and the lower the boiling point and melting point (bottom right hand side of transition metals). As electrons become less delocalized the metals become harder, m ...
STUDY MATERIAL 2015-16 CHEMISTRY CLASS XI
... Kendriya Vidyalaya Sangathan is a pioneer organization which caters to the all round development of the students. Time to time various strategies have been adopted to adorn the students with academic excellence. This support material is one such effort by Kendriya Vidyalaya Sangathan, an empirical e ...
... Kendriya Vidyalaya Sangathan is a pioneer organization which caters to the all round development of the students. Time to time various strategies have been adopted to adorn the students with academic excellence. This support material is one such effort by Kendriya Vidyalaya Sangathan, an empirical e ...
Aldehydes, Ketones and Carboxylic Acids
... The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermediate is produced. This intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bo ...
... The hybridisation of carbon changes from sp2 to sp3 in this process, and a tetrahedral alkoxide intermediate is produced. This intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bo ...
CHANNELING OF SUBSTRATES AND INTERMEDIATES IN
... labile intermediates can be protected from decomposition by the aqueous external environment (7). Unfavorable equilibria can be circumvented (8–10) and reaction intermediates can be segregated from competing enzymatic transformations. Examples of substrate channeling have been cited for numerous bio ...
... labile intermediates can be protected from decomposition by the aqueous external environment (7). Unfavorable equilibria can be circumvented (8–10) and reaction intermediates can be segregated from competing enzymatic transformations. Examples of substrate channeling have been cited for numerous bio ...
Chapter 14: Chemical Kinetics
... For a reaction to occur as a result of a specific collision, the collision must have enough energy to overcome the energy barrier (activation energy). As you saw in Gases (Unit 10) and Intermolecular Forces and the Liquid State (Unit 11), the molecules in a given sample have a Boltzmann distribution ...
... For a reaction to occur as a result of a specific collision, the collision must have enough energy to overcome the energy barrier (activation energy). As you saw in Gases (Unit 10) and Intermolecular Forces and the Liquid State (Unit 11), the molecules in a given sample have a Boltzmann distribution ...
Amines - ncert
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
Practice Exam I FR Answers and Explanations
... (a) Predict sign of Eº and explain. The sign of Eº must be positive. The prompt gives a K value of 1.5 × 1011 which means that the products are favored at equilibrium. Since the reaction proceeds as written, the voltage should be positive. (b) Identify reducing agent. Cd changes oxidation states fro ...
... (a) Predict sign of Eº and explain. The sign of Eº must be positive. The prompt gives a K value of 1.5 × 1011 which means that the products are favored at equilibrium. Since the reaction proceeds as written, the voltage should be positive. (b) Identify reducing agent. Cd changes oxidation states fro ...
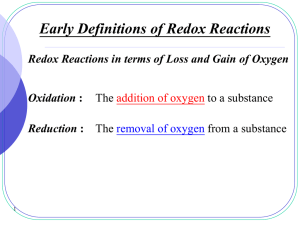
Redox reactions - SALEM-Immanuel Lutheran College
... the oxidation number of an atom in a compound with polar covalent bonds is equal to the charge it would have if it existed as an ion in that compound. E.g. H Cl , since Cl is more electronegative, the presumed electrical charges and thus O.N. of Cl and H are 1 and 1 respectively. ...
... the oxidation number of an atom in a compound with polar covalent bonds is equal to the charge it would have if it existed as an ion in that compound. E.g. H Cl , since Cl is more electronegative, the presumed electrical charges and thus O.N. of Cl and H are 1 and 1 respectively. ...
CHAPTER 3 STOICHIOMETRY:
... Yes; because there are equal numbers of both N and O atoms in the two boxes ...
... Yes; because there are equal numbers of both N and O atoms in the two boxes ...
Exemplar Paper
... During the maturation phase, the grape juice is first fermented in large vats producing ethanol and smaller amounts of higher alcohols such as 3-methylbutan-1-ol and butan-2,3-diol. Exposure to oxygen at this stage causes the yeasts in the grape skins to multiply and allows other chemical reactions ...
... During the maturation phase, the grape juice is first fermented in large vats producing ethanol and smaller amounts of higher alcohols such as 3-methylbutan-1-ol and butan-2,3-diol. Exposure to oxygen at this stage causes the yeasts in the grape skins to multiply and allows other chemical reactions ...
Chem 2A Final Review
... 58. Both water and sulfur dioxide are produced from the reaction of sulfuric acid (H 2SO4) with copper metal in the following balanced equation. How many moles of H2O will be produced at the same time that 10.0 moles of SO2 is produced? 2H2SO4 + Cu SO2 + 2H2O + CuSO4 ...
... 58. Both water and sulfur dioxide are produced from the reaction of sulfuric acid (H 2SO4) with copper metal in the following balanced equation. How many moles of H2O will be produced at the same time that 10.0 moles of SO2 is produced? 2H2SO4 + Cu SO2 + 2H2O + CuSO4 ...
Removal of Chlorine Removal of Chlorine
... Zr(OH)4-based media loaded with TEDA able to effectively remove chlorine gases from streams of air. TEDA is necessary to promote hydrolysis reactions involving Cl2 and COCl2 Terminal hydroxyl groups associated with Zr(OH)4 contribute to the removal of HCl and product HCl (from hydrolysis of Cl2 or C ...
... Zr(OH)4-based media loaded with TEDA able to effectively remove chlorine gases from streams of air. TEDA is necessary to promote hydrolysis reactions involving Cl2 and COCl2 Terminal hydroxyl groups associated with Zr(OH)4 contribute to the removal of HCl and product HCl (from hydrolysis of Cl2 or C ...
contact - DTU Kemi
... ephedrine – all “household names” – are members of the alkaloid family, a group of naturally occurring compounds containing nitrogen atoms. Besides these well-known examples, a large variety of other alkaloids exist. Many of these occur in nature, while others are synthetic, typically produced as dr ...
... ephedrine – all “household names” – are members of the alkaloid family, a group of naturally occurring compounds containing nitrogen atoms. Besides these well-known examples, a large variety of other alkaloids exist. Many of these occur in nature, while others are synthetic, typically produced as dr ...
Kinetics of Oxidation of Benzyl Alcohol with Dilute Nitric Acid
... first studied in detail by Ogata et al.15 They proposed that the reaction proceeded through some intermediate; however, it was not identified. Many other investigators have also studied the oxidation of benzyl alcohol using nitric acid.17-21 However, the published information suffers from two limita ...
... first studied in detail by Ogata et al.15 They proposed that the reaction proceeded through some intermediate; however, it was not identified. Many other investigators have also studied the oxidation of benzyl alcohol using nitric acid.17-21 However, the published information suffers from two limita ...
Quantum Tunnelling to the Origin and Evolution of Life
... of the resulting electrical current. These variations are caused by the local density of electronic states in the substrate surface and/or by the topography of the surface. Atomic resolution of crystalline surfaces is easily possible due to the extreme sensitivity of the measured current on the tip- ...
... of the resulting electrical current. These variations are caused by the local density of electronic states in the substrate surface and/or by the topography of the surface. Atomic resolution of crystalline surfaces is easily possible due to the extreme sensitivity of the measured current on the tip- ...
Enthalpy Barriers for Asymmetric SN2 Alkyl
... method was used to estimate barriers for the reactions between CH3OH2+ with methanol; CH3CH2OH2+ with methanol and ethanol; CH3CH2CH2OH2+ with methanol, ethanol, n-propanol, and acetonitrile; (CH3)2CHOH2+ with 2-propanol; and CH3CNH+ with methanol and ethanol. Another purely experimental approach in ...
... method was used to estimate barriers for the reactions between CH3OH2+ with methanol; CH3CH2OH2+ with methanol and ethanol; CH3CH2CH2OH2+ with methanol, ethanol, n-propanol, and acetonitrile; (CH3)2CHOH2+ with 2-propanol; and CH3CNH+ with methanol and ethanol. Another purely experimental approach in ...
TDB-5: Standards and conventions for TDB publications
... non-standard and should not be used. The treatment of organic ligands poses certain problems due to the complicated composition of most of them. A notation in conformity with the structural features of the molecule may take too much space and is difficult to read, whereas the summation over the atom ...
... non-standard and should not be used. The treatment of organic ligands poses certain problems due to the complicated composition of most of them. A notation in conformity with the structural features of the molecule may take too much space and is difficult to read, whereas the summation over the atom ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound results in an exception to the solubility guidelines. For example, sodium nitrate, NaNO3, is a ...
... compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound results in an exception to the solubility guidelines. For example, sodium nitrate, NaNO3, is a ...
Bulgarian Chemical Communications, Volume 41, Number 4 (pp
... from one phase to another. Hypochlorite oxidations and UV/H2O2 or UV/O3 processes are found to be efficient methods for decolourisation, but they are not desirable due to the high cost of equipment and the secondary pollution arising from the residual chlorine, which further complicates the process. ...
... from one phase to another. Hypochlorite oxidations and UV/H2O2 or UV/O3 processes are found to be efficient methods for decolourisation, but they are not desirable due to the high cost of equipment and the secondary pollution arising from the residual chlorine, which further complicates the process. ...
Review Unit 8 Test (Chp 15,17)
... Greater pressure of reactant initially (Q = 0/1.00 = 0) so forward rate is faster due to greater collision frequency of reactant particles. The forward rate slows over time as reactant is consumed and there is a lower collision frequency of reactant particles, but it does not reach zero b/c more rea ...
... Greater pressure of reactant initially (Q = 0/1.00 = 0) so forward rate is faster due to greater collision frequency of reactant particles. The forward rate slows over time as reactant is consumed and there is a lower collision frequency of reactant particles, but it does not reach zero b/c more rea ...