step by step Stoichiometry
... 2) Write a conversion equation. a) Find the mols of the compound with known mass. b) Use the mol ratio (in the balanced reaction) between the 2 compounds you are interested in. c) Find the grams of the compound you are looking for. **The only time you look at the balanced reaction is for step 2b.!!* ...
... 2) Write a conversion equation. a) Find the mols of the compound with known mass. b) Use the mol ratio (in the balanced reaction) between the 2 compounds you are interested in. c) Find the grams of the compound you are looking for. **The only time you look at the balanced reaction is for step 2b.!!* ...
A Model For the Calculation of Solvent ... Reaction Rates for Process Design Purposes
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
... solvents be chosen with respect not only to their effectiveness in their respective process tasks but also for process-wide requirements such as their ease of recovery, low toxicity and environmental impact and possible applicability to other process tasks. Although there are models for the evaluati ...
updated chem cp final review key
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
... f. Adding an enzyme Increases rate of reaction g. Breaking a reactant into smaller pieces Increases rate of reaction 43. Know what conditions are true of a chemical reaction at equilibrium. (1) rates of forward and reverse reactions are equal. (2) The concentrations of all substances involved stop ...
View/Open
... tutorials based on your level of understanding. Online homework for this chapter may be assigned in Organic OWL. ...
... tutorials based on your level of understanding. Online homework for this chapter may be assigned in Organic OWL. ...
STOICHIOMETRY via ChemLog - Small
... must make the number of blue elements before the reaction equal to the number of blue elements after the reaction. The same must be done for the red elements. Lets first begin with the products of the reaction. In order to make the number of blue blocks before and after the reaction equal, we need t ...
... must make the number of blue elements before the reaction equal to the number of blue elements after the reaction. The same must be done for the red elements. Lets first begin with the products of the reaction. In order to make the number of blue blocks before and after the reaction equal, we need t ...
Final Review 3-8 Answers_2
... 3. A formation reaction is one of several general reaction types. Identify the formation reaction for sulfur dioxode. a) S8(s) + 8 O2(g) 8 SO2(g) b) S(s) + O2(g) SO2(g) c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass o ...
... 3. A formation reaction is one of several general reaction types. Identify the formation reaction for sulfur dioxode. a) S8(s) + 8 O2(g) 8 SO2(g) b) S(s) + O2(g) SO2(g) c) SO2(g) + H2O (l) H2SO3(aq) d) SO2(g) + ½ O2(g) SO3(g) 4. The experimental design of most directly determining the mass o ...
Chemical Reactions and Stoichiometry
... solvent–solute interactions), as shown in Figure 4.1 ▶. For example, when sodium chloride is put into water, there is a competition between the attraction of Na+ cations and Cl- anions to each other (due to their opposite charges) and the attraction of Na+ and Cl- to water molecules. The attraction ...
... solvent–solute interactions), as shown in Figure 4.1 ▶. For example, when sodium chloride is put into water, there is a competition between the attraction of Na+ cations and Cl- anions to each other (due to their opposite charges) and the attraction of Na+ and Cl- to water molecules. The attraction ...
Topic 7.2 Equilibrium The Position of Equilibrium
... The effect of a catalyst on equilibrium Adding a catalyst speeds up a reaction by providing an alternative mechanism with a lower activation energy, thus speeding up both the forward and backward reaction rate. It shortens the time needed to attain equilibrium concentrations It has no effect ...
... The effect of a catalyst on equilibrium Adding a catalyst speeds up a reaction by providing an alternative mechanism with a lower activation energy, thus speeding up both the forward and backward reaction rate. It shortens the time needed to attain equilibrium concentrations It has no effect ...
Document
... • it represents the fraction of reactant molecules with sufficient energy to make it over the energy barrier • that extra energy comes from converting the KE of motion to PE in the molecule when the molecules collide • e-Ea/RT decreases as Ea increases ...
... • it represents the fraction of reactant molecules with sufficient energy to make it over the energy barrier • that extra energy comes from converting the KE of motion to PE in the molecule when the molecules collide • e-Ea/RT decreases as Ea increases ...
EQUILIBRIUM - SCH4U1-CCVI
... (b) What would be the advantages of such a process for commercial production of a substance in industry? ...
... (b) What would be the advantages of such a process for commercial production of a substance in industry? ...
g - mrnicholsscience
... Write the reaction • Butane gas(C4H10) burns in oxygen gas to form carbon dioxide gas and water vapor C4H10(g) + O2(g) CO2(g) + H2O(g) ...
... Write the reaction • Butane gas(C4H10) burns in oxygen gas to form carbon dioxide gas and water vapor C4H10(g) + O2(g) CO2(g) + H2O(g) ...
Chemistry Unit 1
... Acidic oxides are the oxides formed by the chemical combination of oxygen with nonmetals. Thus, acidic oxides are non-metal oxides. These oxides are also called acid anhydrides, since they form acidic solutions when reacted or dissolved in water. Acid anhydride means acid without water. ...
... Acidic oxides are the oxides formed by the chemical combination of oxygen with nonmetals. Thus, acidic oxides are non-metal oxides. These oxides are also called acid anhydrides, since they form acidic solutions when reacted or dissolved in water. Acid anhydride means acid without water. ...
Modern inorganic chemistry
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
... We now know of the existence of over one hundred elements. A century ago, more than sixty of these were already known, and naturally attempts were made to relate the properties of all these elements in some way. One obvious method was to classify them as metals and non-metals; but this clearly did n ...
- Kendriya Vidyalaya Jamuna Colliery
... 5. In terms of band theory what is the difference between a conductor, an insulator and a semiconductor? The energy gap between the valence band and conduction band in an insulator is very large while in a conductor, the energy gap is very small or there is overlapping between valence band and condu ...
... 5. In terms of band theory what is the difference between a conductor, an insulator and a semiconductor? The energy gap between the valence band and conduction band in an insulator is very large while in a conductor, the energy gap is very small or there is overlapping between valence band and condu ...
NICKEL(II) PINCER COMPLEXES SUPPORTED BY 2,6
... metal center, and in some cases incorporating Y groups as well. For example, the abbreviated name for the general structure in Figure 1 would be DXD or DYXYD. Pincer ligands will be explained with this way in this project. ...
... metal center, and in some cases incorporating Y groups as well. For example, the abbreviated name for the general structure in Figure 1 would be DXD or DYXYD. Pincer ligands will be explained with this way in this project. ...
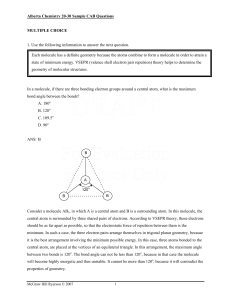
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
... central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between them is the minimum. In such a case, the three electron pairs arrange themselves in trigonal planar geome ...
odd - WWW2
... formed by the most electropositive metals. These may contain the dicarbide(2 ) ion, C22 , or the true carbide ion C4 . Both types of ionic carbides react with water to produce the appropriate hydrocarbon. Covalent carbides are formed by nonmetals, specifically boron and silicon, more electronegative ...
... formed by the most electropositive metals. These may contain the dicarbide(2 ) ion, C22 , or the true carbide ion C4 . Both types of ionic carbides react with water to produce the appropriate hydrocarbon. Covalent carbides are formed by nonmetals, specifically boron and silicon, more electronegative ...
2016-2018 Syllabus - Cambridge International Examinations
... content areas of the syllabus and demonstrate a clear understanding of the relationships between these. Candidates apply knowledge and chemical principles contained within the syllabus in both familiar and unfamiliar contexts. In questions requiring numerical calculations, candidates demonstrate goo ...
... content areas of the syllabus and demonstrate a clear understanding of the relationships between these. Candidates apply knowledge and chemical principles contained within the syllabus in both familiar and unfamiliar contexts. In questions requiring numerical calculations, candidates demonstrate goo ...
Chemical Reactions - 2012 Book Archive
... Chapter 2 "Molecules, Ions, and Chemical Formulas" introduced you to a wide variety of chemical compounds, many of which have interesting applications. For example, nitrous oxide, a mild anesthetic, is also used as the propellant in cans of whipped cream, while copper(I) oxide is used as both a red ...
... Chapter 2 "Molecules, Ions, and Chemical Formulas" introduced you to a wide variety of chemical compounds, many of which have interesting applications. For example, nitrous oxide, a mild anesthetic, is also used as the propellant in cans of whipped cream, while copper(I) oxide is used as both a red ...
(III) ion and a cobalt (II) - Iowa State University Digital Repository
... data and the curves drawn through the experimental data are the final best fit (calculated with the numerical ...
... data and the curves drawn through the experimental data are the final best fit (calculated with the numerical ...
Chemical Reaction Equations
... Reactions are fast – the reaction must occur within a reasonable time (see pg. 280) Reactions are quantitative – one that is more than 99% complete; in other words, at least one reactant is completely used up Reactions are stoichiometric – means that there is a simple whole-number ratio of chemical ...
... Reactions are fast – the reaction must occur within a reasonable time (see pg. 280) Reactions are quantitative – one that is more than 99% complete; in other words, at least one reactant is completely used up Reactions are stoichiometric – means that there is a simple whole-number ratio of chemical ...
Chapter 6 Chemical Reactions
... (c) The amount of the diammine complex, Ag(NH3)2+(aq), increases with time as more and more AgCl(s) and NH3(aq) react. Problem 11.8. Molecules move faster at higher temperatures. (Their internal motions (vibrations and rotations) also gain more energy, so vibrations and rotations are more active.) M ...
... (c) The amount of the diammine complex, Ag(NH3)2+(aq), increases with time as more and more AgCl(s) and NH3(aq) react. Problem 11.8. Molecules move faster at higher temperatures. (Their internal motions (vibrations and rotations) also gain more energy, so vibrations and rotations are more active.) M ...
Class-XII, Summer assignment
... 2. PH3 has lower boiling point than NH3. Why? Ans: Unlike NH3, PH3 molecules are not associated through hydrogen bonding in liquid state. That is why the boiling point of PH3 is lower than NH3. 3. Why are pentahalides more covalent than trihalides ? Ans: Higher the positive oxidation state of centra ...
... 2. PH3 has lower boiling point than NH3. Why? Ans: Unlike NH3, PH3 molecules are not associated through hydrogen bonding in liquid state. That is why the boiling point of PH3 is lower than NH3. 3. Why are pentahalides more covalent than trihalides ? Ans: Higher the positive oxidation state of centra ...