Quantitative Comparison of the Hydrogen Bond
... FIGURE 1: Hydrogen bond topologies as derived by detected h3JNC′ H-bond correlations for ubiquitin in the native state (A) and in the A-state (B). Top: two-dimensional maps representing the detected h3JNC′ scalar correlations (filled diamonds). Secondary structure elements are indicated as filled ar ...
... FIGURE 1: Hydrogen bond topologies as derived by detected h3JNC′ H-bond correlations for ubiquitin in the native state (A) and in the A-state (B). Top: two-dimensional maps representing the detected h3JNC′ scalar correlations (filled diamonds). Secondary structure elements are indicated as filled ar ...
text
... We use a single arrow ( $ ) in place of the equilibrium arrow ( ? ) be‑ cause we treat HCl as if it dissociates completely in an aqueous solution. In water, the common strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), nitric acid (HNO3), perchloric acid (HClO4), ...
... We use a single arrow ( $ ) in place of the equilibrium arrow ( ? ) be‑ cause we treat HCl as if it dissociates completely in an aqueous solution. In water, the common strong acids are hydrochloric acid (HCl), hydroiodic acid (HI), hydrobromic acid (HBr), nitric acid (HNO3), perchloric acid (HClO4), ...
Chapter 16 Controlling the yield of reactions
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
... a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0.44 mol of H2 and 0.44 mol of I2 were present. Calculate the value of the equilibrium constant at this temperature. c A third mixture consisted of ...
AQA A-level Chemistry
... A reaction profile is a diagram of the enthalpy levels of the reactants and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the ...
... A reaction profile is a diagram of the enthalpy levels of the reactants and products in a chemical reaction. The vertical (y) axis is enthalpy but not ∆H. The horizontal (x) axis is progress of reaction, reaction coordinate or extent of reaction. Two horizontal lines are drawn and labelled with the ...
Homework extension
... http://www.docbrown.info/page01/ExIndChem/electrochemistry04.htm oxidised. When you do electrolysis with solutions: At the negative electrode: Metal will be produced on the electrode if it is less reactive than hydrogen. Hydrogen will be produced if the metal is more reactive than hydrogen. At the p ...
... http://www.docbrown.info/page01/ExIndChem/electrochemistry04.htm oxidised. When you do electrolysis with solutions: At the negative electrode: Metal will be produced on the electrode if it is less reactive than hydrogen. Hydrogen will be produced if the metal is more reactive than hydrogen. At the p ...
Chemical Vapor Deposition (CVD)
... coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total pressure gas flows, etc.— materials with different pr ...
... coatings, powders, fibers and monolithic components. • This technique is often used in many thin film applications. • By varying the experimental conditions—substrate material, substrate temperature, composition of the reaction gas mixture, total pressure gas flows, etc.— materials with different pr ...
عرض تقديمي من PowerPoint
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
... Heterocyclic analogues of Cyclopropane All three membered rings have one major property in common- a strained ring which confers great reactivity on the compounds in comparison with their openchain analogues. ...
Surface and sub-surface reactions during low temperature
... This study focuses on chemical reactions that occur upon vapor-phase metal-organic precursor exposure during lowtemperature atomic layer deposition on reactive and nonreactive polymer surfaces. Several common polymers with different ...
... This study focuses on chemical reactions that occur upon vapor-phase metal-organic precursor exposure during lowtemperature atomic layer deposition on reactive and nonreactive polymer surfaces. Several common polymers with different ...
Acid-Base Biochemistry
... why some substances, such as common baking soda (NaHCO3), can act like a base even though they do not contain hydroxide ions. ...
... why some substances, such as common baking soda (NaHCO3), can act like a base even though they do not contain hydroxide ions. ...
Equilibrium
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
... The value of the equilibrium constant for any reaction can be determined by experiment. As detailed in the above section, the equilibrium position for a given reaction does not depend on the starting concentrations, so the equilibrium constant has the same value regardless of the initial amounts of ...
Exam Review Packet Table of Contents
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
Problem 1-2 - IPN-Kiel
... iv) Calcium Which acid/base properties do the aqueous solutions of the products show? ...
... iv) Calcium Which acid/base properties do the aqueous solutions of the products show? ...
A* PLC Legacy GCSE Chemistry (all boards)
... http://www.docbrown.info/page01/ExIndChem/electrochemistry04.htm oxidised. When you do electrolysis with solutions: At the negative electrode: Metal will be produced on the electrode if it is less reactive than hydrogen. Hydrogen will be produced if the metal is more reactive than hydrogen. At the p ...
... http://www.docbrown.info/page01/ExIndChem/electrochemistry04.htm oxidised. When you do electrolysis with solutions: At the negative electrode: Metal will be produced on the electrode if it is less reactive than hydrogen. Hydrogen will be produced if the metal is more reactive than hydrogen. At the p ...
Ch 10 Practice Problems 1. Consider the process A(l) A(s). Which
... As O2(l) is cooled at 1 atm, it freezes at 54.5 K to form Solid I. At a lower temperature, Solid I rearranges to Solid II, which has a different crystal structure. Thermal measurements show that H for the I II phase transition is –743.1 J/mol and that S for the same transition is –17.0 J/K mol. ...
... As O2(l) is cooled at 1 atm, it freezes at 54.5 K to form Solid I. At a lower temperature, Solid I rearranges to Solid II, which has a different crystal structure. Thermal measurements show that H for the I II phase transition is –743.1 J/mol and that S for the same transition is –17.0 J/K mol. ...
AS Chemistry Teacher Handbook
... Metallic bonding should be considered as attraction between positive ions and a delocalised ‘electron ...
... Metallic bonding should be considered as attraction between positive ions and a delocalised ‘electron ...
Document
... the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex ion, [Cu(NH3)4]2+. Complex ions may be positively charged cations or neg ...
... the most interesting ions have a metal ion surrounded by a number of ligands. Ligands are molecules, such as ammonia, NH3, or anions, such as cyanide, CN −, that readily bond to metal ions. Figure 5 shows a model of one complex ion, [Cu(NH3)4]2+. Complex ions may be positively charged cations or neg ...
Chapter 8 "Ionic versus Covalent Bonding"
... mol of positive and negative ions condenses to form a crystalline lattice. Because of long-range interactions in the lattice structure, this energy does not correspond directly to the lattice energy of the crystalline solid. However, the large negative value indicates that bringing positive and nega ...
... mol of positive and negative ions condenses to form a crystalline lattice. Because of long-range interactions in the lattice structure, this energy does not correspond directly to the lattice energy of the crystalline solid. However, the large negative value indicates that bringing positive and nega ...
Synthesis and Structural Studies of Calcium and Magnesium
... growth including hydrothermal methods and gel crystallization were employed. We have used phosphinate and phosphonate ligands with different number of phosphorus oxygen atoms as well as diphosphonates with different linker lengths to determine their effects on the overall structural features. An int ...
... growth including hydrothermal methods and gel crystallization were employed. We have used phosphinate and phosphonate ligands with different number of phosphorus oxygen atoms as well as diphosphonates with different linker lengths to determine their effects on the overall structural features. An int ...
Organic Acids and Bases and Some of Their Derivatives
... found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group. A carboxylic acid1 is an organic compound that has a carboxyl group2. The carboxyl group is a functional group that contains a carbon–oxygen double bond and an OH grou ...
... found in carboxylic acids, esters, and amides. However, in these compounds, the carbonyl group is only part of the functional group. A carboxylic acid1 is an organic compound that has a carboxyl group2. The carboxyl group is a functional group that contains a carbon–oxygen double bond and an OH grou ...
"Cyano Compounds, Inorganic," in: Ullmann`s Encyclopedia of
... Chemical Properties. Some comprehensive reviews on hydrogen cyanide and cyanogen compounds have been published [1–3]. The acid occurs only in the nitrile form. Although isomeric isonitrile HNC has been detected in interstellar space, all efforts to isolate this compound have failed. As the nitrile o ...
... Chemical Properties. Some comprehensive reviews on hydrogen cyanide and cyanogen compounds have been published [1–3]. The acid occurs only in the nitrile form. Although isomeric isonitrile HNC has been detected in interstellar space, all efforts to isolate this compound have failed. As the nitrile o ...
Carbon dioxide capture and utilization in petrochemical industry
... mixture by fractional condensation and distillation at low temperature. Cryogenic temperatures are obtained by a closed-cycle operated refrigeration system consisting of a compressor, a Joule-Thompson valve (JTV), multi-stage heat exchangers and expanders. Cryogenic distillation is a commercial proc ...
... mixture by fractional condensation and distillation at low temperature. Cryogenic temperatures are obtained by a closed-cycle operated refrigeration system consisting of a compressor, a Joule-Thompson valve (JTV), multi-stage heat exchangers and expanders. Cryogenic distillation is a commercial proc ...
Brilliant Preparatory Section, Sitamarhi
... This skeleton equation itself is a balanced one. But in many cases the skeleton equation is not a balanced one. For example, the decomposition of Lead Nitrate giving Lead oxide, NO2 and oxygen. The skeletal equation for this reaction is Pb(NO3)2 → PbO + NO2 + O2 iv. In the skeleton equation, the num ...
... This skeleton equation itself is a balanced one. But in many cases the skeleton equation is not a balanced one. For example, the decomposition of Lead Nitrate giving Lead oxide, NO2 and oxygen. The skeletal equation for this reaction is Pb(NO3)2 → PbO + NO2 + O2 iv. In the skeleton equation, the num ...
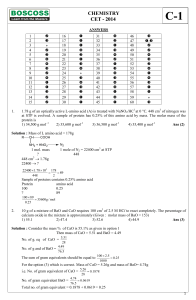
CHEMISTRY CET
... Volume of 0.03 M KMnO4 required to oxidize the same amount of H2S gas to sulphur, in acidic medium is 1) 80 cm3 2) 120 cm3 3) 60 cm3 4) 90 cm3 Solution : K2Cl2O4 KMnO4 ...
... Volume of 0.03 M KMnO4 required to oxidize the same amount of H2S gas to sulphur, in acidic medium is 1) 80 cm3 2) 120 cm3 3) 60 cm3 4) 90 cm3 Solution : K2Cl2O4 KMnO4 ...
Chm 2
... d. 11.0 mol For the reaction represented by the equation Cl2 + 2KBr 2KCl + Br2, how many moles of potassium chloride are produced from 119 g of potassium bromide? a. 0.119 mol c. 0.581 mol b. 0.236 mol d. 1.00 mol For the reaction represented by the equation 3Fe + 4H2O Fe3O4 + 4H2, how many mole ...
... d. 11.0 mol For the reaction represented by the equation Cl2 + 2KBr 2KCl + Br2, how many moles of potassium chloride are produced from 119 g of potassium bromide? a. 0.119 mol c. 0.581 mol b. 0.236 mol d. 1.00 mol For the reaction represented by the equation 3Fe + 4H2O Fe3O4 + 4H2, how many mole ...