Kinetics in the Study of Organic Reaction Mechanisms
... nature must be deduced from indirect evidence of various kinds. The most powerful tool for the experimental study of reaction mechanisms is chemical Kinetics. Kinetics deals with the rates at which chemical reactions occur, and with all of the factors which influence these rates, No reaction mechani ...
... nature must be deduced from indirect evidence of various kinds. The most powerful tool for the experimental study of reaction mechanisms is chemical Kinetics. Kinetics deals with the rates at which chemical reactions occur, and with all of the factors which influence these rates, No reaction mechani ...
FYBSc Revised Syllabus
... solubility in water; van der Waals forces. 1.6 Structure of common functional groups: geometry and electronic structure in order to understand their reactivity. 2. Nomenclature of organic compounds: 7L 2.1 Functional groups and types of organic compounds, basic rules of IUPAC nomenclature. 2.2 Nomen ...
... solubility in water; van der Waals forces. 1.6 Structure of common functional groups: geometry and electronic structure in order to understand their reactivity. 2. Nomenclature of organic compounds: 7L 2.1 Functional groups and types of organic compounds, basic rules of IUPAC nomenclature. 2.2 Nomen ...
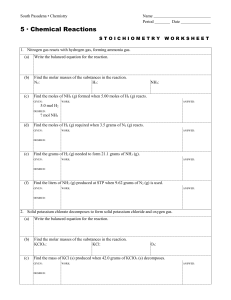
South Pasadena • Chemistry Name Period Date 5 · Chemical
... 4. A solution of lead acetate is combined with a solution of hydrochloric acid forming a lead chloride precipitate and acetic acid. (a) Write the balanced equation for the reaction. ...
... 4. A solution of lead acetate is combined with a solution of hydrochloric acid forming a lead chloride precipitate and acetic acid. (a) Write the balanced equation for the reaction. ...
Wk-11-14
... Wheels + Pedals + Handlebar Bicycle Unbalanced: a list of ingredients & results 2 Wheels + 2 Pedals + 1 Handlebar Bicycle Balanced: a correct recipe ...
... Wheels + Pedals + Handlebar Bicycle Unbalanced: a list of ingredients & results 2 Wheels + 2 Pedals + 1 Handlebar Bicycle Balanced: a correct recipe ...
Practice Test 1 (Chapters 1-7)
... the smallest possible integers, what is the number in front of the substance in bold type? NBr3 + NaOH N2 + NaBr + HOBr a. b. c. d. e. ...
... the smallest possible integers, what is the number in front of the substance in bold type? NBr3 + NaOH N2 + NaBr + HOBr a. b. c. d. e. ...
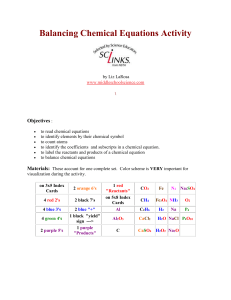
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
Chemistry
... Crystallization and decolorisation of impure naphthalene (100g of naphthalene mixes with 0.3 g of Congo Red using 1g decolorizing carbon) from ethanol. ...
... Crystallization and decolorisation of impure naphthalene (100g of naphthalene mixes with 0.3 g of Congo Red using 1g decolorizing carbon) from ethanol. ...
Chapter 11: Reactions of Alkyl Halides There are two basic types of
... TsO⊖, MsO⊖ > I⊖ > Br⊖ > Cl⊖ ≈ H2O > F⊖, ⊖OH, ⊖NH2 SN1 reactions are often run under acidic conditions, therefore H2O is often seen as a Leaving Group for alcohols (refer to Ch 10, formation of tertiary halides from tertiary ...
... TsO⊖, MsO⊖ > I⊖ > Br⊖ > Cl⊖ ≈ H2O > F⊖, ⊖OH, ⊖NH2 SN1 reactions are often run under acidic conditions, therefore H2O is often seen as a Leaving Group for alcohols (refer to Ch 10, formation of tertiary halides from tertiary ...
2 KClO 3
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
... Wheels + Pedals + Handlebar ---» Bicycle Unbalanced: a list of ingredients & results ...
do not
... Inhibitors are chemicals which reduce the rate of enzyme catalyzed reactions – Alter the shape of the active site directly or indirectly 1)Non-reversible bind permanently disabling enzyme – Permanent change of tertiary structure (break disulfide bond) ...
... Inhibitors are chemicals which reduce the rate of enzyme catalyzed reactions – Alter the shape of the active site directly or indirectly 1)Non-reversible bind permanently disabling enzyme – Permanent change of tertiary structure (break disulfide bond) ...
Surface chemistry Surface chemistry deals with phenomena that
... The reactants must get adsorbed reasonably strongly on to the catalyst to become active. However, they must not get adsorbed so strongly that they are immobilised and other reactants are left with no space on the catalyst’s surface for adsorption. (b) Selectivity The selectivity of a catalyst is its ...
... The reactants must get adsorbed reasonably strongly on to the catalyst to become active. However, they must not get adsorbed so strongly that they are immobilised and other reactants are left with no space on the catalyst’s surface for adsorption. (b) Selectivity The selectivity of a catalyst is its ...
do not - wwphs
... Inhibitors are chemicals which reduce the rate of enzyme catalyzed reactions – Alter the shape of the active site directly or indirectly 1)Non-reversible bind permanently disabling enzyme – Permanent change of tertiary structure (break disulfide bond) ...
... Inhibitors are chemicals which reduce the rate of enzyme catalyzed reactions – Alter the shape of the active site directly or indirectly 1)Non-reversible bind permanently disabling enzyme – Permanent change of tertiary structure (break disulfide bond) ...
Heat of reaction
... to make informed decisions. • For several reactions a direct measurement can be done with a calorimeter. • Many times this is impossible or it is a time consuming task which makes it very hard. • Hess’s law allows us to manipulate equations for calculating ΔH for a given reaction. • If a reaction is ...
... to make informed decisions. • For several reactions a direct measurement can be done with a calorimeter. • Many times this is impossible or it is a time consuming task which makes it very hard. • Hess’s law allows us to manipulate equations for calculating ΔH for a given reaction. • If a reaction is ...
AP Chemistry Syllabus 2013 Mawhiney
... 4. Solve problems using Faraday's law. 5. Predict reaction products for both electrolytic and voltaic cells. 6. Use a table of Standard Reduction Potentials to compute cell voltages. 7. Diagram voltaic cells using proper notation. 8. Establish the relationship between the free energy change, the cel ...
... 4. Solve problems using Faraday's law. 5. Predict reaction products for both electrolytic and voltaic cells. 6. Use a table of Standard Reduction Potentials to compute cell voltages. 7. Diagram voltaic cells using proper notation. 8. Establish the relationship between the free energy change, the cel ...
Chemistry 199 - Oregon State chemistry
... Yes, water acts like a Lewis base—especially in transition metal chemistry. The lone pairs of electrons on the oxygen atom are donated to a metal ion to form a new bond. ...
... Yes, water acts like a Lewis base—especially in transition metal chemistry. The lone pairs of electrons on the oxygen atom are donated to a metal ion to form a new bond. ...
2. Essential Chemistry
... o Acids - substances that able to ionize in solution to form hydrogen ion (H+) and increase the concentration of H+ in the solution. For example, HCl dissociate in water to form H+ and Cl- ions. o Bases - are substances that can react with or accept H+ ions. For example, OH- will accept H+ from ...
... o Acids - substances that able to ionize in solution to form hydrogen ion (H+) and increase the concentration of H+ in the solution. For example, HCl dissociate in water to form H+ and Cl- ions. o Bases - are substances that can react with or accept H+ ions. For example, OH- will accept H+ from ...
Unit 3 Ch. 9 - Classifying Chemical Reactions
... North American Indians: Find the “Origins aof Navaho Silver” display. Note that the belt ornaments are slightly tarnished. What is tarnshing?! When silver tarnishes, it combines with sulfur and forms silver sulfide (Ag2S). Silver sulfide is black. When a thin coating of silver sulfide forms on the s ...
... North American Indians: Find the “Origins aof Navaho Silver” display. Note that the belt ornaments are slightly tarnished. What is tarnshing?! When silver tarnishes, it combines with sulfur and forms silver sulfide (Ag2S). Silver sulfide is black. When a thin coating of silver sulfide forms on the s ...
Microbial Biogeochemistry
... (chemolithoheterotrophs) • Examples, Thiobacillus sp. And Beggiatoa sp. • Methanotrophs: CH4 + O2 CO2 + 2H2O (chemoorganoheterotrophs) • Example, Ralstonia sp., Pseudomonas sp. Anaerobic Environment Fermentors (chemoorganoheterotrophs) • Break down cellulose, etc. and ferment sugars into: • alcoho ...
... (chemolithoheterotrophs) • Examples, Thiobacillus sp. And Beggiatoa sp. • Methanotrophs: CH4 + O2 CO2 + 2H2O (chemoorganoheterotrophs) • Example, Ralstonia sp., Pseudomonas sp. Anaerobic Environment Fermentors (chemoorganoheterotrophs) • Break down cellulose, etc. and ferment sugars into: • alcoho ...
EXAM 1 - gozips.uakron.edu
... ___ Al 4 C 3 (s) + ___ H 2 O (l) → ___ CH 4 (g) + ___ Al(OH) 3 (s) (A) 1 (B) 3 (C) 4 (D) 6 (E) 12 ...
... ___ Al 4 C 3 (s) + ___ H 2 O (l) → ___ CH 4 (g) + ___ Al(OH) 3 (s) (A) 1 (B) 3 (C) 4 (D) 6 (E) 12 ...
chemical reaction - Peoria Public Schools
... chemical reaction that uses symbols to show the relationship between the reactants and products ...
... chemical reaction that uses symbols to show the relationship between the reactants and products ...
lecture slides of chap19_FU
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Chemistry 1 - Edexcel
... (i) Place a tick (9) in one box in each row of the table to show the best method of separation for each mixture. ...
... (i) Place a tick (9) in one box in each row of the table to show the best method of separation for each mixture. ...
Reaction Rate review questions
... State the collision theory for chemical reactions. The Collision Theory states: 1. Reacting particles must collide with each other. In order to react, reactant particles must collide with each other. 2. The reactant particles must collide hard enough to break old chemical bonds so that new chemical ...
... State the collision theory for chemical reactions. The Collision Theory states: 1. Reacting particles must collide with each other. In order to react, reactant particles must collide with each other. 2. The reactant particles must collide hard enough to break old chemical bonds so that new chemical ...
Chapter 2: Chemical Basis of Life
... It would be difficult to appreciate fully the characteristics of living matter and its functions without looking at the basic principles of chemistry as they apply to life processes. In fact, it is almost impossible to speak of either the components or the processes of living things without using th ...
... It would be difficult to appreciate fully the characteristics of living matter and its functions without looking at the basic principles of chemistry as they apply to life processes. In fact, it is almost impossible to speak of either the components or the processes of living things without using th ...