Redox - edl.io
... 5. Oxygen is usually assigned an oxidation state of -2. Exceptions to this rule include peroxides (compound containing the O22- group), where each oxygen is assigned an oxidation state of -1, as in hydrogen peroxide (H2O2), and OF2 in which oxygen is assigned a +2 oxidation state. 6. In its covalent ...
... 5. Oxygen is usually assigned an oxidation state of -2. Exceptions to this rule include peroxides (compound containing the O22- group), where each oxygen is assigned an oxidation state of -1, as in hydrogen peroxide (H2O2), and OF2 in which oxygen is assigned a +2 oxidation state. 6. In its covalent ...
Practice Exam #2
... is __________, and therefore heat is __________ by the reaction. A) exothermic, released B) exothermic, absorbed C) endothermic, absorbed D) endothermic, released E) thermoneutral, neither released nor absorbed 17) The value of ΔE for a system that performs 213 kJ of work on its surroundings and los ...
... is __________, and therefore heat is __________ by the reaction. A) exothermic, released B) exothermic, absorbed C) endothermic, absorbed D) endothermic, released E) thermoneutral, neither released nor absorbed 17) The value of ΔE for a system that performs 213 kJ of work on its surroundings and los ...
The Effect of Solvent on a Lewis Acid Catalyzed Diels− Alder
... (Figure 1) shows that the chlorine atom is only 2.87 Å from ...
... (Figure 1) shows that the chlorine atom is only 2.87 Å from ...
Selective Ethanol Synthesis from Carbon Dioxide
... Work on the synthesis of ethanol from carbon dioxide over a rhodiumselenium catalyst i s reported, and related reactions and characterisation studies are briefly reviewed. I n order to inhibit theformation of methane (complete reduction of carbon dioxide) and simultaneously activate carbon-carbon bo ...
... Work on the synthesis of ethanol from carbon dioxide over a rhodiumselenium catalyst i s reported, and related reactions and characterisation studies are briefly reviewed. I n order to inhibit theformation of methane (complete reduction of carbon dioxide) and simultaneously activate carbon-carbon bo ...
(null): 110.ReactionsIntro
... "nitric acid acts upon copper." I was getting tired of reading such absurd stuff and I was determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked nitric acid on a table in the doctor's office where I was then "doing ti ...
... "nitric acid acts upon copper." I was getting tired of reading such absurd stuff and I was determined to see what this meant. Copper was more or less familiar to me, for copper cents were then in use. I had seen a bottle marked nitric acid on a table in the doctor's office where I was then "doing ti ...
7.2: Properties, Names, and Formulas page 268 •Acids and bases
... 7.2: Properties, Names, and Formulas ...
... 7.2: Properties, Names, and Formulas ...
Types of Chemical Reactions
... nonelectrolytes – solutions where dissolving has occurred but the solute does not make ions and therefore cannot conduct electricity. (Pure water, sugar, alcohols, antifreeze, and starch) ...
... nonelectrolytes – solutions where dissolving has occurred but the solute does not make ions and therefore cannot conduct electricity. (Pure water, sugar, alcohols, antifreeze, and starch) ...
Take Home - mvhs
... suspected of being this illicit drug; when tested, the sample was shown to have a percentage composition of 83.71% C, 10.42% H, and 5.61% N. Prove if this sample is phencyclidine. (2 pts work, 1 pt correct answer, 1 pt correct sig figs) ...
... suspected of being this illicit drug; when tested, the sample was shown to have a percentage composition of 83.71% C, 10.42% H, and 5.61% N. Prove if this sample is phencyclidine. (2 pts work, 1 pt correct answer, 1 pt correct sig figs) ...
Empirical is the
... 75. Many homes in rural America are heated by propane gas, a compound that contains only carbon and hydrogen. Complete combustion of a sample of propane produced 2.641 g of Carbon dioxide and 1.442 g of water as the only products. Find the empirical formula of propane. Remember that a combustion re ...
... 75. Many homes in rural America are heated by propane gas, a compound that contains only carbon and hydrogen. Complete combustion of a sample of propane produced 2.641 g of Carbon dioxide and 1.442 g of water as the only products. Find the empirical formula of propane. Remember that a combustion re ...
DEPARTMENT OF CHEMISTRY
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
... a. Write the reactions (total of 5) for each of the secondary, tertiary, and aryl substrates listed in 1.e. above with ethanol and silver nitrate in the table on the next page. b. Obtain 5 clean, dry, new test tubes (10 x 75 mm size) and parafilm. Devise a scheme to enable you to keep track of each ...
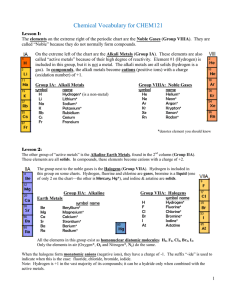
Vocabulary CHEM121
... Salts: all other ionic materials Formulas of molecular/covalent compounds: Non-metals can combine with other non-metals to form molecules. Molecules are covalently bonded groups of atoms—they do not have a charge like polyatomic ions, but are neutral. Since more than one combination is possible, the ...
... Salts: all other ionic materials Formulas of molecular/covalent compounds: Non-metals can combine with other non-metals to form molecules. Molecules are covalently bonded groups of atoms—they do not have a charge like polyatomic ions, but are neutral. Since more than one combination is possible, the ...
Dendrimer-Encapsulated Pd Nanoparticles as Aqueous, Room
... after treatment with MSTFA. The mass spectrum of each peak confirmed that it corresponds to the trimethylsilyl derivatives (See Supporting Information). Figure 2c is a chromatogram of the derivatized crude product resulting from the DEN-catalyzed Stille reaction. The retention time and mass spectrum ...
... after treatment with MSTFA. The mass spectrum of each peak confirmed that it corresponds to the trimethylsilyl derivatives (See Supporting Information). Figure 2c is a chromatogram of the derivatized crude product resulting from the DEN-catalyzed Stille reaction. The retention time and mass spectrum ...
Section 1 The Nature of Chemical Reactions
... Classifying Reactions, continued • A single-displacement reaction is a reaction in which one element or radical takes the place of another element or radical in the compound. • Single-displacement reactions have the following general form: AX + B → BX + A • Example: The single-displacement reaction ...
... Classifying Reactions, continued • A single-displacement reaction is a reaction in which one element or radical takes the place of another element or radical in the compound. • Single-displacement reactions have the following general form: AX + B → BX + A • Example: The single-displacement reaction ...
Physical and Chemical change: Introduction
... test tube and add a few drops of 6 M HCl. NOTE: Be very careful when you handle this acid because it can cause major burns. 4. Take about 0,5 g iron lings and 0,5 g sulphur. Test each substance with a magnet. Mix the two samples in a test tube and run a magnet alongside the outside of the test tube ...
... test tube and add a few drops of 6 M HCl. NOTE: Be very careful when you handle this acid because it can cause major burns. 4. Take about 0,5 g iron lings and 0,5 g sulphur. Test each substance with a magnet. Mix the two samples in a test tube and run a magnet alongside the outside of the test tube ...
Chem 106 Week 10.2017
... Write a complete balanced chemical equation for the reaction by adding products and stoichiometric coefficients from the above reactants. ...
... Write a complete balanced chemical equation for the reaction by adding products and stoichiometric coefficients from the above reactants. ...
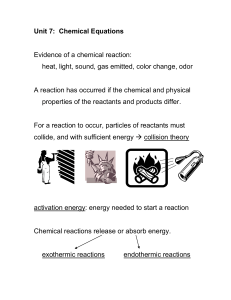
chemical reaction?
... • What is an exothermic reaction? – A chemical reaction in which energy is released to the surroundings – Exothermic reactions often feel __________ because energy is released as heat – An example of an exothermic reaction is _______________ ...
... • What is an exothermic reaction? – A chemical reaction in which energy is released to the surroundings – Exothermic reactions often feel __________ because energy is released as heat – An example of an exothermic reaction is _______________ ...
Unit 9 The p-Block Elements
... The high reactivity of fluorine is explained partly in terms of its low bond dissociation enthalpy, which means that little energy is required to break the F-F bond in the initial stages of a reaction. Another factor is the tendency to form strong bonds with other elements. Thus, fluorine tends to b ...
... The high reactivity of fluorine is explained partly in terms of its low bond dissociation enthalpy, which means that little energy is required to break the F-F bond in the initial stages of a reaction. Another factor is the tendency to form strong bonds with other elements. Thus, fluorine tends to b ...
the optimization of proton exchange membrane hydrogen fuel cells
... A catalyst that has ideal selectivity minimizes the number of intermediates and unwanted waste products in the reactions at the anode and cathode [9]. The hydrogen oxidation has only one possible mechanism and therefore the selectivity does not affect the anode [9]. There are two possible mechanisms ...
... A catalyst that has ideal selectivity minimizes the number of intermediates and unwanted waste products in the reactions at the anode and cathode [9]. The hydrogen oxidation has only one possible mechanism and therefore the selectivity does not affect the anode [9]. There are two possible mechanisms ...
PEA: Chemistry: Mole City Worksheet
... More Mole City 4. How many grams of the aqueous product would be formed in double replacement reaction when 10.0 grams of aqueous barium chloride is reacted with an excess amount of aqueous silver nitrate. ...
... More Mole City 4. How many grams of the aqueous product would be formed in double replacement reaction when 10.0 grams of aqueous barium chloride is reacted with an excess amount of aqueous silver nitrate. ...
Chemistry - Nagpur University
... (B) Liquid-Liquid mixtures : Ideal liquid mixtures, Raoults law of ideal solutions, Henry’s law, nonideal systems, azeotropes : HCl-H2O & ethanol- water system. Partial miscible liquids : phenol-water system, trimethylamine-water, nicotine-water system, lower & upper consolute temperature, effect of ...
... (B) Liquid-Liquid mixtures : Ideal liquid mixtures, Raoults law of ideal solutions, Henry’s law, nonideal systems, azeotropes : HCl-H2O & ethanol- water system. Partial miscible liquids : phenol-water system, trimethylamine-water, nicotine-water system, lower & upper consolute temperature, effect of ...
Organic Chemistry I: Contents
... Importance of Pi bond in organic compounds: • Pi bond has slightly higher energy (less stable) than sigma bond. The bond dissociation energy of sigma bond in ethylene molecule is account to be 95 kcal/mol, while Pi bond is 68 kcal/mol. • The Pi bond is polarized more easily, it’s delocalized bond ( ...
... Importance of Pi bond in organic compounds: • Pi bond has slightly higher energy (less stable) than sigma bond. The bond dissociation energy of sigma bond in ethylene molecule is account to be 95 kcal/mol, while Pi bond is 68 kcal/mol. • The Pi bond is polarized more easily, it’s delocalized bond ( ...
ouble Replacement or (Metathesis) Reactions
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
Synthesis of Alum Lab
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
... 2Al(s) + 2K+(aq) + 2OH-(aq) + 6H2O(l) 2[Al(OH)4]-(aq) + 2K+(aq) + 3H2(g) The oxidation and reduction half reactions: ...
Types of Chemical Reactions
... show different colors above and below the indicator’s pH constant. • The color and the pH range it changes colors at vary for different indicators. • The most common indicator, phenolphthalein, turns pink in basic solutions (pH > 7) and clear in acidic solutions (pH < 7). ...
... show different colors above and below the indicator’s pH constant. • The color and the pH range it changes colors at vary for different indicators. • The most common indicator, phenolphthalein, turns pink in basic solutions (pH > 7) and clear in acidic solutions (pH < 7). ...