Chemical reactions unit
... There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so the particles have more opportu ...
... There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so the particles have more opportu ...
SAT Practice Test 3
... Benzene has delocalized pi electrons that stabilize its structure The element with an electron configuration of [He]2s 1 has a greater nuclear charge than fluorine 1 mole of NaCl yields 3 moles of ions in solution Neutrons and protons are both located in the principal energy levels of the atom HCl i ...
... Benzene has delocalized pi electrons that stabilize its structure The element with an electron configuration of [He]2s 1 has a greater nuclear charge than fluorine 1 mole of NaCl yields 3 moles of ions in solution Neutrons and protons are both located in the principal energy levels of the atom HCl i ...
Chemical reactions unit
... There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so the particles have more opportu ...
... There are 6 factors that affect the rate of chemical reactions: 1. Increase in temperature: Why? The particles are moving faster and have more chances to collide into each other to make a reaction. 2. Increase in Surface area: Why? More of the substance is exposed, so the particles have more opportu ...
H 2 O
... • When an atom or molecule loses electrons, it becomes positively charged. – For example, when Na loses an electron it becomes Na+. • Positively charged ions are called cations. • When an atom or molecule gains electrons, it becomes negatively charged. • For example when Cl gains an electron it beco ...
... • When an atom or molecule loses electrons, it becomes positively charged. – For example, when Na loses an electron it becomes Na+. • Positively charged ions are called cations. • When an atom or molecule gains electrons, it becomes negatively charged. • For example when Cl gains an electron it beco ...
Class: 11 Subject: Chemistry Topic: Equilibrium No. of
... 11. Assertion (A) The pH of an aqueous solution of acetic acid remains unchanged on the addition of sodium acetate. Reason (R) The ionisation of acetic acid is suppressed by the addition of sodium acetate. A. Both (A) and (R) are true and (R) is the correct explanation of (A). B. Both (A) and (R) ar ...
... 11. Assertion (A) The pH of an aqueous solution of acetic acid remains unchanged on the addition of sodium acetate. Reason (R) The ionisation of acetic acid is suppressed by the addition of sodium acetate. A. Both (A) and (R) are true and (R) is the correct explanation of (A). B. Both (A) and (R) ar ...
Complex Ions and Free Energy
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly ...
... form between metal ions and ligands. Furthermore, I can determine the coordination number for a coordination complex • LT 8.7 – I can calculate the formation constant for complex ions and relate that to the Ksp for a slightly ...
File
... Emission spectra are produced when photons are emitted from atoms as excited electrons return to a lower energy level The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies The main energy level or shell is ...
... Emission spectra are produced when photons are emitted from atoms as excited electrons return to a lower energy level The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies The main energy level or shell is ...
with answers
... reaction proceeds. Why was it such a great challenge to develop an efficient industrial synthesis of ammonia, for which Carl Bosch obtained the Nobel Prize? N2 + 3 H2 2 NH3 The entropy decreases, as there are fewer gas molecules on the right-hand side. Very high activation energy, so rate of uncatal ...
... reaction proceeds. Why was it such a great challenge to develop an efficient industrial synthesis of ammonia, for which Carl Bosch obtained the Nobel Prize? N2 + 3 H2 2 NH3 The entropy decreases, as there are fewer gas molecules on the right-hand side. Very high activation energy, so rate of uncatal ...
synoptic - chemnotes.org.uk
... A covalent bond is a shared pair of electrons Covalent bonds hold atoms together because both nuclei are attracted to the shared pair of electrons The strength of the bond depends on the strength of attraction between the nuclei and the shared pair Down a group attraction for the shared pair will de ...
... A covalent bond is a shared pair of electrons Covalent bonds hold atoms together because both nuclei are attracted to the shared pair of electrons The strength of the bond depends on the strength of attraction between the nuclei and the shared pair Down a group attraction for the shared pair will de ...
full text - pdf 452 kB
... The AH and AC' values for reactions (6) and (7) are shown in Fig. 5 (1 1, 14). The AH values do not change significantly with temperature and the AC, values are small for reactions (6) and (7). The AC,, AH, and AS values for the association of K+, Ba2+, and Sr2+ with 18-crown-6 (18C6) are large and ...
... The AH and AC' values for reactions (6) and (7) are shown in Fig. 5 (1 1, 14). The AH values do not change significantly with temperature and the AC, values are small for reactions (6) and (7). The AC,, AH, and AS values for the association of K+, Ba2+, and Sr2+ with 18-crown-6 (18C6) are large and ...
Unit 7 Packet
... When you heated sodium hydrogen carbonate, you decomposed it into sodium oxide, water vapor, and gaseous carbon dioxide. ...
... When you heated sodium hydrogen carbonate, you decomposed it into sodium oxide, water vapor, and gaseous carbon dioxide. ...
Development of Novel Catalytic Asymmetric Reactions using
... As described in Scheme 5, we found that protonation can significantly enhance the reactivity of imines. Therefore, encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophil ...
... As described in Scheme 5, we found that protonation can significantly enhance the reactivity of imines. Therefore, encouraged by the cooperative action presented in Scheme 9, we also investigated a Mannich-type reaction employing imine, which exhibits high affinity to protic acid, as the electrophil ...
organic chemistry - Peoria Public Schools
... carbon-carbon bond and is broken much easier. It is because of this greater reactivity that alkenes, especially ethene, are important starting materials in organic synthesis of useful chemicals. It is important to note that alkenes also easily combust and undergo both complete and incomplete combust ...
... carbon-carbon bond and is broken much easier. It is because of this greater reactivity that alkenes, especially ethene, are important starting materials in organic synthesis of useful chemicals. It is important to note that alkenes also easily combust and undergo both complete and incomplete combust ...
File
... increased, the equilibrium position shifts to use up the heat by producing more products. (moves to right) In an exothermic reaction, energy can be considered as a product of the reaction. If the temperature of an exothermic equilibrium system is increased, the equilibrium position shifts to use up ...
... increased, the equilibrium position shifts to use up the heat by producing more products. (moves to right) In an exothermic reaction, energy can be considered as a product of the reaction. If the temperature of an exothermic equilibrium system is increased, the equilibrium position shifts to use up ...
View Article - Asian Journal of Chemistry
... s, 1021 m, 859 m, 766 w, 730 w, 674 w, 567 w. 1H NMR (400 MHz, CDCl3): δ 1.30 (t, 3H), 1.83 (d, 3H), 4.23 (m, 2H), 4.36 (t, 1H). The Hell-Volhard-Zelinsky reaction is synthetically useful, since it gives rise to α-brominated products which the bromine atom can be easily replaced to form other functi ...
... s, 1021 m, 859 m, 766 w, 730 w, 674 w, 567 w. 1H NMR (400 MHz, CDCl3): δ 1.30 (t, 3H), 1.83 (d, 3H), 4.23 (m, 2H), 4.36 (t, 1H). The Hell-Volhard-Zelinsky reaction is synthetically useful, since it gives rise to α-brominated products which the bromine atom can be easily replaced to form other functi ...
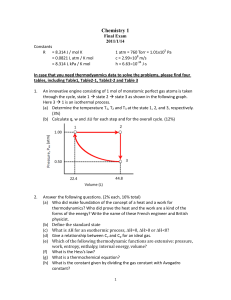
Chemistry 1
... (b) Define the standard state (c) What is H for an exothermic process, H=0, H>0 or H<0? (d) Give a relationship between Cv and Cp for an ideal gas. (e) Which of the following thermodynamic functions are extensive: pressure, work, entropy, enthalpy, internal energy, volume? (f) What is the Hess’s ...
... (b) Define the standard state (c) What is H for an exothermic process, H=0, H>0 or H<0? (d) Give a relationship between Cv and Cp for an ideal gas. (e) Which of the following thermodynamic functions are extensive: pressure, work, entropy, enthalpy, internal energy, volume? (f) What is the Hess’s ...
CHEM1100 Practice Exam 2 You have 120 minutes to complete this
... 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl–, Br–, I– are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. 4. Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually a ...
... 2. Salts containing nitrate ion (NO3-) are generally soluble. 3. Salts containing Cl–, Br–, I– are generally soluble. Important exceptions to this rule are halide salts of Ag+, Pb2+, and (Hg2)2+. 4. Most silver salts are insoluble. AgNO3 and Ag(C2H3O2) are common soluble salts of silver; virtually a ...
Chemical Equations
... the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
... the arrow) and the products (on the right of the arrow). C. The law of conservation of mass and energy must be satisfied. Therefore the same number of atoms of each element must appear on each side of a correct chemical equation. ...
Click Here To File
... solution whereas the isomer [Co(NH3)5SO4]Br does not form this precipitate. (or any other relevant test) (a) KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through car ...
... solution whereas the isomer [Co(NH3)5SO4]Br does not form this precipitate. (or any other relevant test) (a) KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through car ...
Summer Work
... 3. The number of protons in one atom of an element determines the atom’s __________________ , and the number of electrons determines ___________________ of an element. 4. The atomic number tells you the number of ______________________ in one atom of an element. It also tells you the number of _____ ...
... 3. The number of protons in one atom of an element determines the atom’s __________________ , and the number of electrons determines ___________________ of an element. 4. The atomic number tells you the number of ______________________ in one atom of an element. It also tells you the number of _____ ...
1 H NT Ch 12—Stoichiometry I. Review: Chemical Equations a
... ii. Carbon tetrachloride was prepared by reacting 100.0 g of carbon disulfide with 100.0 grams of chlorine gas. Calculate the theoretical and percent yield if 65.0 g of carbon tetrachloride was obtained. U ...
... ii. Carbon tetrachloride was prepared by reacting 100.0 g of carbon disulfide with 100.0 grams of chlorine gas. Calculate the theoretical and percent yield if 65.0 g of carbon tetrachloride was obtained. U ...