Chemistry - Solutions
... • Solubility: the amount of a substance that will dissolve in a given amount of solvent to form a saturated solution at a given temperature ...
... • Solubility: the amount of a substance that will dissolve in a given amount of solvent to form a saturated solution at a given temperature ...
doc
... I. Organic Molecules A. Inorganic Compounds – compounds not formed by living things (usually don’t contain carbon – exception is carbon dioxide) B .Organic – Organic means ‘made by living things.’ Most organic compounds contain carbon. Carbon has four electrons in its valence cloud, wants to form 4 ...
... I. Organic Molecules A. Inorganic Compounds – compounds not formed by living things (usually don’t contain carbon – exception is carbon dioxide) B .Organic – Organic means ‘made by living things.’ Most organic compounds contain carbon. Carbon has four electrons in its valence cloud, wants to form 4 ...
The light reaction of photosynthesis does not include
... photosynthesis occurs only in autotrophs; cellular respiration occurs only in A) heterotrophs photosynthesis uses solar energy to convert inorganics to energy-rich organics; B) respiration breaks down energy-rich organics to synthesize ATP photosynthesis involves the oxidation of glucose; respiratio ...
... photosynthesis occurs only in autotrophs; cellular respiration occurs only in A) heterotrophs photosynthesis uses solar energy to convert inorganics to energy-rich organics; B) respiration breaks down energy-rich organics to synthesize ATP photosynthesis involves the oxidation of glucose; respiratio ...
Topic guide 1.1: Amino acids and proteins
... Amino acids join together when a condensation reaction occurs between the acid group of one amino acid and the amino group of another amino acid. A covalent bond is formed between the two amino acids and a water molecule is produced. The bond that forms between the two amino acids is known as a ...
... Amino acids join together when a condensation reaction occurs between the acid group of one amino acid and the amino group of another amino acid. A covalent bond is formed between the two amino acids and a water molecule is produced. The bond that forms between the two amino acids is known as a ...
Chemistry of Life
... oxygen in exact ratios (CnH2nOn) Carbs are divided into two classes called simple sugars (monosaccharides and disaccharides) and complex sugars (oligosaccharides and polysaccharides). ...
... oxygen in exact ratios (CnH2nOn) Carbs are divided into two classes called simple sugars (monosaccharides and disaccharides) and complex sugars (oligosaccharides and polysaccharides). ...
Introduction to Biochemistry
... oxygen in exact ratios (CnH2nOn) Carbs are divided into two classes called simple sugars (monosaccharides and disaccharides) and complex sugars (oligosaccharides and polysaccharides). ...
... oxygen in exact ratios (CnH2nOn) Carbs are divided into two classes called simple sugars (monosaccharides and disaccharides) and complex sugars (oligosaccharides and polysaccharides). ...
Name 1 BIO 451 14 December, 1998 FINAL EXAM
... Starch is the plant equivalent of glycogen; the linkages among the sugars are identical but starch is less highly branched. What is the principle reason that marathon runners would be better advised to eat pasta (high starch content) rather than a meal containing a high amount of sucrose prior to pa ...
... Starch is the plant equivalent of glycogen; the linkages among the sugars are identical but starch is less highly branched. What is the principle reason that marathon runners would be better advised to eat pasta (high starch content) rather than a meal containing a high amount of sucrose prior to pa ...
Enzymes
... 1.absorb and distribute materials 2.obtain and hydrolyze materials 3.release energy from food 4.produce cellular waste products ...
... 1.absorb and distribute materials 2.obtain and hydrolyze materials 3.release energy from food 4.produce cellular waste products ...
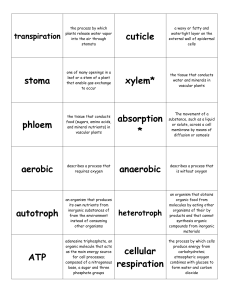
Ecology
... an organism that produces its own nutrients from inorganic substances of from the environment instead of consuming other organisms ...
... an organism that produces its own nutrients from inorganic substances of from the environment instead of consuming other organisms ...
Experimentally testing the hypothesis of a limited amino acid
... types of amino acids were involved in the earliest protein synthesis system? By comparing homologous amino acid sequences from different organisms or referring to various criteria for amino acid chronology, chronological order of appearance of amino acids in the early evolution have been predicted [ ...
... types of amino acids were involved in the earliest protein synthesis system? By comparing homologous amino acid sequences from different organisms or referring to various criteria for amino acid chronology, chronological order of appearance of amino acids in the early evolution have been predicted [ ...
Chapter Nine
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
Lecture 6
... Protein structure: Amino acids • Essential vs non-essential – Essential: NOT made by body – Nonessential: made by the body ...
... Protein structure: Amino acids • Essential vs non-essential – Essential: NOT made by body – Nonessential: made by the body ...
Flexibility of a polypeptide chain
... phi and psi values actually limit the number of possible structures and it is overcome by interactions that favor the folded form e.g. hydrophobic interactions among apolar side-chains (rather than being exposed to polar water), H-bonding network, S-S bridges, ion pairs, etc. a highly flexible polym ...
... phi and psi values actually limit the number of possible structures and it is overcome by interactions that favor the folded form e.g. hydrophobic interactions among apolar side-chains (rather than being exposed to polar water), H-bonding network, S-S bridges, ion pairs, etc. a highly flexible polym ...
CHAPTER 9
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
File
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
chapter 9
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
... cell where each stage occurs. 8. Describe how the carbon skeleton of glucose changes as it proceeds through glycolysis. 9. Explain why ATP is required for the preparatory steps of glycolysis. 10. Identify where substrate-level phosphorylation and the reduction of NAD+ occur in glycolysis. 11. Descri ...
Document
... oxidation of carbs, protein and fatty acids, are ultimately transferred to O2 to produce H20 Located in the inner mitochondrial membrane Electrons travel down the chain, pumping protons into the intermembrane space creating the driving force to produce ATP in a process called oxidative phosphory ...
... oxidation of carbs, protein and fatty acids, are ultimately transferred to O2 to produce H20 Located in the inner mitochondrial membrane Electrons travel down the chain, pumping protons into the intermembrane space creating the driving force to produce ATP in a process called oxidative phosphory ...
Amino Acids - Biology Learning Center
... mRNA: messenger RNA; RNA string copied (‘transcribed’) from DNA tRNA: transfer RNA; one of many RNA molecules that carry specific amino acids ribosome: giant machine (>200 proteins, 4 RNAs (2 > 1000 nucleotides) that oversees the reading of the mRNA and the creation of polypeptide aminoacyl tRNA syn ...
... mRNA: messenger RNA; RNA string copied (‘transcribed’) from DNA tRNA: transfer RNA; one of many RNA molecules that carry specific amino acids ribosome: giant machine (>200 proteins, 4 RNAs (2 > 1000 nucleotides) that oversees the reading of the mRNA and the creation of polypeptide aminoacyl tRNA syn ...
Extension and Enrichment
... Examine the amino acid chart provided and review the properties of the different amino acids. 1. Hydrophobic side chains (R) primarily contain _________________ atoms. ...
... Examine the amino acid chart provided and review the properties of the different amino acids. 1. Hydrophobic side chains (R) primarily contain _________________ atoms. ...
Lecture 8 LC710- 1st + 2nd hr
... If there was a single tRNATyr gene, then could one have a amber supressor of it? ...
... If there was a single tRNATyr gene, then could one have a amber supressor of it? ...
- Free Documents
... process of selective transport. Trypsinogen is converted into active trypsin by the enzyme enterokinase. The major proteolytic enzyme in the stomach is pepsin. while carboxypeptidase B cleaves Cterminal arginine or lysine residues. and proelastase. Pepsin preferentially attacks peptide bonds involvi ...
... process of selective transport. Trypsinogen is converted into active trypsin by the enzyme enterokinase. The major proteolytic enzyme in the stomach is pepsin. while carboxypeptidase B cleaves Cterminal arginine or lysine residues. and proelastase. Pepsin preferentially attacks peptide bonds involvi ...
File
... would cause the amount of oxygen in the atmosphere to decrease (deforestation). Plants produce oxygen during photosynthesis. The amount of Carbon Dioxide would also increase because there would not be any plants using the CO2 in the atmosphere (as they do for photosynthesis). 7. Why is solar energy ...
... would cause the amount of oxygen in the atmosphere to decrease (deforestation). Plants produce oxygen during photosynthesis. The amount of Carbon Dioxide would also increase because there would not be any plants using the CO2 in the atmosphere (as they do for photosynthesis). 7. Why is solar energy ...
Primary Structure
... the compositions are seen. The high amount of Serine is worth noting. This could have an effect on the kinetics of the antibody fragment binding, or for potential glycosylation if found at the surface. High Serine levels allow the protein to be soluble. Also worth noting is that there is twice as ma ...
... the compositions are seen. The high amount of Serine is worth noting. This could have an effect on the kinetics of the antibody fragment binding, or for potential glycosylation if found at the surface. High Serine levels allow the protein to be soluble. Also worth noting is that there is twice as ma ...
Biochemistry
_and_Carl_Ferdinand_Cori.jpg?width=300)
Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last decades of the 20th century, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine to genetics are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Biochemistry is closely related to molecular biology, the study of the molecular mechanisms by which genetic information encoded in DNA is able to result in the processes of life. Depending on the exact definition of the terms used, molecular biology can be thought of as a branch of biochemistry, or biochemistry as a tool with which to investigate and study molecular biology.Much of biochemistry deals with the structures, functions and interactions of biological macromolecules, such as proteins, nucleic acids, carbohydrates and lipids, which provide the structure of cells and perform many of the functions associated with life. The chemistry of the cell also depends on the reactions of smaller molecules and ions. These can be inorganic, for example water and metal ions, or organic, for example the amino acids which are used to synthesize proteins. The mechanisms by which cells harness energy from their environment via chemical reactions are known as metabolism. The findings of biochemistry are applied primarily in medicine, nutrition, and agriculture. In medicine, biochemists investigate the causes and cures of disease. In nutrition, they study how to maintain health and study the effects of nutritional deficiencies. In agriculture, biochemists investigate soil and fertilizers, and try to discover ways to improve crop cultivation, crop storage and pest control.