Lec-23_Strachan
... stimulated absorption and stimulated emission are equally probable Generally, a net absorption occurs since most atoms are in the ground state If you can cause more atoms to be in excited states, a net emission of photons can result This situation is called a population inversion ...
... stimulated absorption and stimulated emission are equally probable Generally, a net absorption occurs since most atoms are in the ground state If you can cause more atoms to be in excited states, a net emission of photons can result This situation is called a population inversion ...
A short description how to calculate absorption and energy transfer
... the method, it is applied to study optical absorption and the coherent-incoherent transition of energy transfer in ringshaped molecular aggregates [6], as they appear, e.g., in the light-harvesting units of some bacteria. We consider QAs where the wave functions of different monomers do not overlap ...
... the method, it is applied to study optical absorption and the coherent-incoherent transition of energy transfer in ringshaped molecular aggregates [6], as they appear, e.g., in the light-harvesting units of some bacteria. We consider QAs where the wave functions of different monomers do not overlap ...
MS WORD - Rutgers Physics
... qualitative features of the global phase diagram by mapping the classical spin model into the single-layer quantum Ising model. In the 3D limit ( N ), the two upper BKT transitions collapse into a single 3D-XY critical point that signals the transition into a partially disordered antiferromagneti ...
... qualitative features of the global phase diagram by mapping the classical spin model into the single-layer quantum Ising model. In the 3D limit ( N ), the two upper BKT transitions collapse into a single 3D-XY critical point that signals the transition into a partially disordered antiferromagneti ...
D - The University of British Columbia
... Crystal with tunable impurities: • Time-domain quantum simulation of localization of quantum particles: timescale of Anderson localization dynamics of exciton localization as a function of effective mass, exciton ...
... Crystal with tunable impurities: • Time-domain quantum simulation of localization of quantum particles: timescale of Anderson localization dynamics of exciton localization as a function of effective mass, exciton ...
Instrumental Methods of Analysis
... Quantum Mechanical Model of Atomic Movement only certain energies are allowed. near equilibrium position, molecular vibrations similar to mechanical model.. Frequency of the vibration from the mechanical model as a reasonable estimate of true vibrational frequency: . ...
... Quantum Mechanical Model of Atomic Movement only certain energies are allowed. near equilibrium position, molecular vibrations similar to mechanical model.. Frequency of the vibration from the mechanical model as a reasonable estimate of true vibrational frequency: . ...
Quantum theory
... momentum of an e- at the same time • Scientists are unable to describe the exact structure of an atom due to this • But it can be determined with probability • Can determine with high probability where an e- is most likely to be found in the energy levels of an atom at any one given time ...
... momentum of an e- at the same time • Scientists are unable to describe the exact structure of an atom due to this • But it can be determined with probability • Can determine with high probability where an e- is most likely to be found in the energy levels of an atom at any one given time ...
A computer program to calculate the total energy absorption cross
... the failure of the complex absorbing potential to fully absorb the wavepacket incident on it. This may lead to both reflected or transmitted waves. The reader should consult the two papers by Vibók and Balint-Kurti [9,10] for the optimal choices of parameters for the complex absorbing potential. ...
... the failure of the complex absorbing potential to fully absorb the wavepacket incident on it. This may lead to both reflected or transmitted waves. The reader should consult the two papers by Vibók and Balint-Kurti [9,10] for the optimal choices of parameters for the complex absorbing potential. ...
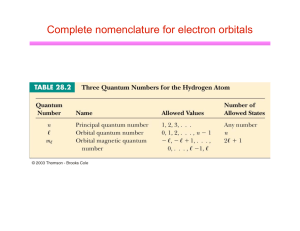
Laboratory Exercise: The Electronic Structure of the Hydrogen Atom
... Electromagnetic Spectrum of photon wavelengths is represented below: ...
... Electromagnetic Spectrum of photon wavelengths is represented below: ...
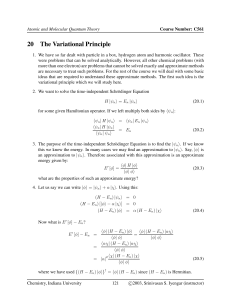
Final Review
... atoms? When an electron undergoes a transition from an orbital to a different orbital this also means that it may undergo a change in energy state as well. It undergoes a change of energy E and discards the excess energy (or absorbs the necessary energy) as a photon of electromagnetic energy with a ...
... atoms? When an electron undergoes a transition from an orbital to a different orbital this also means that it may undergo a change in energy state as well. It undergoes a change of energy E and discards the excess energy (or absorbs the necessary energy) as a photon of electromagnetic energy with a ...
Synthesis and Size Dependent Properties of CdSe Quantum Dots
... case you may navigate to the location of the tool through its link http://www.nanohub.org/tools/cndo. It will take you to the main window of the program; you may read here a brief summary about it and find useful bibliographic references one should use to document the work in which this tool has bee ...
... case you may navigate to the location of the tool through its link http://www.nanohub.org/tools/cndo. It will take you to the main window of the program; you may read here a brief summary about it and find useful bibliographic references one should use to document the work in which this tool has bee ...
1s + 2p
... A time-dependent wavefunction looks just like the spatial s we have been talking about, except that it is multiplied by eit = cos(t) + i sin(t), where i = (-1), is the energy (in frequency units) of the spatial wavefunction and t is time. In many cases this makes no difference, because whe ...
... A time-dependent wavefunction looks just like the spatial s we have been talking about, except that it is multiplied by eit = cos(t) + i sin(t), where i = (-1), is the energy (in frequency units) of the spatial wavefunction and t is time. In many cases this makes no difference, because whe ...
Atomic Term Symbols
... respectively, 9 states in total. A 2 D level (S=1/2, L=2), splits into J=5/2 (6 states) and J=3/2 (4 states), hence 10 states in total. The atomic term values are 2 D5/ 2 , 2 D3/ 2 . You may notice that the splittings discussed for the hydrogen atom follow this rule also. The energy splittings betwe ...
... respectively, 9 states in total. A 2 D level (S=1/2, L=2), splits into J=5/2 (6 states) and J=3/2 (4 states), hence 10 states in total. The atomic term values are 2 D5/ 2 , 2 D3/ 2 . You may notice that the splittings discussed for the hydrogen atom follow this rule also. The energy splittings betwe ...
HS-PS1-6
... HS-PS1-5: Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs. [Clarification Statement: Emphasis is on student reasoning that focuses on the number and ener ...
... HS-PS1-5: Apply scientific principles and evidence to provide an explanation about the effects of changing the temperature or concentration of the reacting particles on the rate at which a reaction occurs. [Clarification Statement: Emphasis is on student reasoning that focuses on the number and ener ...
tutorial13forweek7
... a. Symmetric stretch mode only b. Degenerate bending modes only c. Asymmetric and degenerate bending modes d. Symmetric and asymmetric stretching modes Answer: a 4. The zero point vibrational energy of carbon dioxide is given as a. ...
... a. Symmetric stretch mode only b. Degenerate bending modes only c. Asymmetric and degenerate bending modes d. Symmetric and asymmetric stretching modes Answer: a 4. The zero point vibrational energy of carbon dioxide is given as a. ...
Franck–Condon principle
The Franck–Condon principle is a rule in spectroscopy and quantum chemistry that explains the intensity of vibronic transitions. Vibronic transitions are the simultaneous changes in electronic and vibrational energy levels of a molecule due to the absorption or emission of a photon of the appropriate energy. The principle states that during an electronic transition, a change from one vibrational energy level to another will be more likely to happen if the two vibrational wave functions overlap more significantly.











![ABSTRACT – Condensed Matter Physics [ORIGINAL]](http://s1.studyres.com/store/data/005325689_1-bd59cbe3830dc734895532d6f7679a5c-300x300.png)