department of chemistry ch 102 (inorganic): tutorial no. 5
... 1. The following complexes have the indicated effective magnetic moments. Describe the structure and bonding of the complexes on the basis of the meff values. K2NiF6 0.0; Ni(NH3)2Cl2 3.3; Ni(PEt3)2Cl2 0.0; Ni(Ph3AsO)2Cl2 3.95. ...
... 1. The following complexes have the indicated effective magnetic moments. Describe the structure and bonding of the complexes on the basis of the meff values. K2NiF6 0.0; Ni(NH3)2Cl2 3.3; Ni(PEt3)2Cl2 0.0; Ni(Ph3AsO)2Cl2 3.95. ...
Selection Rules for electronic transitions
... Goals: Use Beers Law to calculate Abs; convert wavelength into wavenumbers; Derive ground state term symbols Upcoming: 11/18, 11/21: Ch. 11. Electronic transitions in metal complexes 11/23 no class 11/28, 11/30: Acc. Chem. Res. 2003, 36, 876-887 Photochemistry for solar energy 12/2: Exam III ...
... Goals: Use Beers Law to calculate Abs; convert wavelength into wavenumbers; Derive ground state term symbols Upcoming: 11/18, 11/21: Ch. 11. Electronic transitions in metal complexes 11/23 no class 11/28, 11/30: Acc. Chem. Res. 2003, 36, 876-887 Photochemistry for solar energy 12/2: Exam III ...
Ligand Field Theory in the New Millenium: Is there Life after DFT?
... d Orbital splittings • In octahedral symmetry, the five d orbitals split • Barycentre relative to average d orbital energy eg ...
... d Orbital splittings • In octahedral symmetry, the five d orbitals split • Barycentre relative to average d orbital energy eg ...
Document
... As Cr3+ goes from being attached to a weak field ligand to a strong field ligand, increases and the color of the complex changes from green to yellow. ...
... As Cr3+ goes from being attached to a weak field ligand to a strong field ligand, increases and the color of the complex changes from green to yellow. ...
Crystal field theory
... Therefore, there is a large, unfavorable interaction between ligand (-) orbitals. These orbitals form the degenerate high energy pair of energy levels. The dxy , dyx and dxz orbitals bisect the negative charges. ...
... Therefore, there is a large, unfavorable interaction between ligand (-) orbitals. These orbitals form the degenerate high energy pair of energy levels. The dxy , dyx and dxz orbitals bisect the negative charges. ...
Structural Preferences of N-Substituted Monosaccharide Derivatives
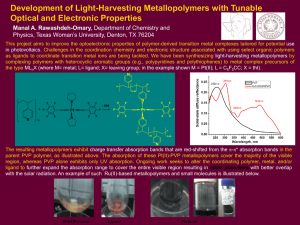
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
... Physics, Texas Woman’s University, Denton, TX 76204 This project aims to improve the optoelectronic properties of polymer-derived transition metal complexes tailored for potential use in photovoltaics. Challenges in the coordination chemistry and electronic structure associated with using select org ...
AP Notes Chapter 11
... The electrostatic field or ligand field exists when the atom or ion is approached by a legand. Electrons lose degeneracy and are pushed into higher and lower spins split by energy ...
... The electrostatic field or ligand field exists when the atom or ion is approached by a legand. Electrons lose degeneracy and are pushed into higher and lower spins split by energy ...
InorgCh11.2
... a. Free Ion (no ligand field) Term Symbols (Previous Lecture) shown on left b. Strong Ligand Field Term Symbols shown on the right: overcomes LS coupling a. Possible d2 electron configurations (t2g2 is ground state lowest energy) ...
... a. Free Ion (no ligand field) Term Symbols (Previous Lecture) shown on left b. Strong Ligand Field Term Symbols shown on the right: overcomes LS coupling a. Possible d2 electron configurations (t2g2 is ground state lowest energy) ...
EXAMINING THE IMPACT OF LIGAND BASICITY ON THE REACTIVITY OF TRANSITION METAL SYSTEMS THROUGH COMPUTATIONAL METHODS
... the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunately, a detailed understanding of the fundamental impact of ligand basicity on a metal c ...
... the properties and observed reactivity of transition metal complexes. Indeed, gaining the ability to “tune” the properties of metal complexes is a primary goal in inorganic and organometallic chemistry. Unfortunately, a detailed understanding of the fundamental impact of ligand basicity on a metal c ...
Inorganic Chemistry review sheet Exam #3 Ch. 9 Lewis acids (e
... Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy." Can influence symmetry (will see one point group via crystallography instead of ano ...
... Jahn Teller effect: “Any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy." Can influence symmetry (will see one point group via crystallography instead of ano ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).