2010 Midterm 2 KEY
... between molecules in the solid state), rationalize the observed change in the magnetic moment with temperature. The complex also shows large changes in its UV/Vis spectrum as a function of temperature. HINT: consider what the moment is telling you about the number of unpaired spins. [4 pts] If the s ...
... between molecules in the solid state), rationalize the observed change in the magnetic moment with temperature. The complex also shows large changes in its UV/Vis spectrum as a function of temperature. HINT: consider what the moment is telling you about the number of unpaired spins. [4 pts] If the s ...
Problem Set 7 - Bryn Mawr College
... c. Ni(CO)4 as a Td complex. Ni(0) is d10, so the Td crystal field splitting with T2 orbitals higher than E orbitals has all these filled. d. [Ni(Cl)4]2- as a D4h complex This was a mistake!! Ni(II) is d8, Cl- is a weak field ligand so its actual structure is Td, not square planar. I believe I intend ...
... c. Ni(CO)4 as a Td complex. Ni(0) is d10, so the Td crystal field splitting with T2 orbitals higher than E orbitals has all these filled. d. [Ni(Cl)4]2- as a D4h complex This was a mistake!! Ni(II) is d8, Cl- is a weak field ligand so its actual structure is Td, not square planar. I believe I intend ...
Key Concepts PowerPoint
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
... Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the +x and +y axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
... 14. Explain the working principle of dye-sensitized solar cells. 15. The epr spectrum of bis(salicylaldimine)copper(II) consists of four sets of eleven lines each. Explain the spectral features. Substantiate your interpretation with experimental evidences. 16. How do you differentiate [Co(en)3]3+ an ...
Schedule • Last Week: Electronic spectroscopy • Last Week
... • explain the number of bands • obtain Δoct from spectrum for d1, d3, d4, d6, d7, d8 and d9 • predict relative intensity of spin-allowed vs spin forbidden octahedral vs tetrahedral and ligand forbidden, ...
... • explain the number of bands • obtain Δoct from spectrum for d1, d3, d4, d6, d7, d8 and d9 • predict relative intensity of spin-allowed vs spin forbidden octahedral vs tetrahedral and ligand forbidden, ...
Experiment VIII
... Coordination compounds of transition metals are often highly colored. The color results from absorption of light at specific wavelengths of visible light associated with electronic transitions within the d-orbitals. Thus, these d-d transitions give many transition metal ions their characteristic col ...
... Coordination compounds of transition metals are often highly colored. The color results from absorption of light at specific wavelengths of visible light associated with electronic transitions within the d-orbitals. Thus, these d-d transitions give many transition metal ions their characteristic col ...
1 HW 5 CHEM 362 Answer Key Available: March
... The Jahn-Teller effect says that degeneracy of a given configuration must be broken by geometric distortion of the molecule to result in non-degenerate configurations. A classic example is a Ti3+ molecule which had is d1 in an ‘octahedral’ ligand set. The single electron would reside in the t2g leve ...
... The Jahn-Teller effect says that degeneracy of a given configuration must be broken by geometric distortion of the molecule to result in non-degenerate configurations. A classic example is a Ti3+ molecule which had is d1 in an ‘octahedral’ ligand set. The single electron would reside in the t2g leve ...
Coordination Chemistry III: Tanabe
... Jahn-Teller Effect in Spectroscopy Frontier MO diagram eg CuII, d9 t2g There should only be one d–d transition, why is there a split in the absorption band at 900 nm? ...
... Jahn-Teller Effect in Spectroscopy Frontier MO diagram eg CuII, d9 t2g There should only be one d–d transition, why is there a split in the absorption band at 900 nm? ...
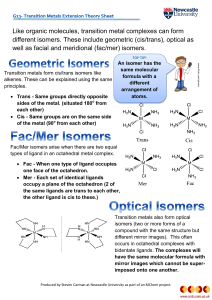
Like organic molecules, transition metal complexes can form
... Transition metals also form optical isomers (two or more forms of a compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto ...
... Transition metals also form optical isomers (two or more forms of a compound with the same structure but different mirror images). This often occurs in octahedral complexes with bidentate ligands. The complexes will have the same molecular formula with mirror images which cannot be superimposed onto ...
Chem212,Quiz5,99
... 4. [2 x 2.5 = 5 points] Explain with reference to ligand field theory how any two of the following three types of ligands affect the crystal field splitting of the d orbitals in an octahedral complex. π-donor ligand - donor ligand π-acceptor ligand In your answers discuss the type of orbital ...
... 4. [2 x 2.5 = 5 points] Explain with reference to ligand field theory how any two of the following three types of ligands affect the crystal field splitting of the d orbitals in an octahedral complex. π-donor ligand - donor ligand π-acceptor ligand In your answers discuss the type of orbital ...
Assignment 6
... • Arrive at your metal d electron count of a complex carefully by considering anionic ligands and overall charge. For example Fe 3+ is 3d5, Mn2+ 3d5 etc • While determining pairing energy P in CFSE always compare with the situation before the splitting • Significant Jahn Teller distortion means dege ...
... • Arrive at your metal d electron count of a complex carefully by considering anionic ligands and overall charge. For example Fe 3+ is 3d5, Mn2+ 3d5 etc • While determining pairing energy P in CFSE always compare with the situation before the splitting • Significant Jahn Teller distortion means dege ...
Experiment 4 Spectroscopic study of Cu(II) Complexes: Crystal Field
... Spectroscopic study of Cu(II) Complexes: Crystal Field Theory Objective: To study the effect of ligands on crystal field splitting energy (E) ...
... Spectroscopic study of Cu(II) Complexes: Crystal Field Theory Objective: To study the effect of ligands on crystal field splitting energy (E) ...
29th Annual Meeting | American Society of Preventive Oncology
... as well as candidate chemotherapy agents. The initial phase of development of these materials is based on the electronic signaling properties of these complexes characterized by electrochemical impedance spectroscopy, and microscopy of their liquid crystal state. Use of a transition metal or a lanth ...
... as well as candidate chemotherapy agents. The initial phase of development of these materials is based on the electronic signaling properties of these complexes characterized by electrochemical impedance spectroscopy, and microscopy of their liquid crystal state. Use of a transition metal or a lanth ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).