Coordination Chemistry II: Theories of Electronic
... Crystal Field Theory – an electrostatic approach to understanding the electronic spectroscopy of crystals – metal valence electrons are perturbed by negative point charges arranged in a regular coordination geometry – no description of M–L bonding ...
... Crystal Field Theory – an electrostatic approach to understanding the electronic spectroscopy of crystals – metal valence electrons are perturbed by negative point charges arranged in a regular coordination geometry – no description of M–L bonding ...
Crystal field theory states that d or f orbital degeneracy
... will have a higher energy than those further away, which results in the d orbitals splitting in energy. This splitting is affected by: the nature of the metal ion the metal's oxidation state (a higher oxidation state leads to a larger splitting) the arrangement of the ligands around the metal ion th ...
... will have a higher energy than those further away, which results in the d orbitals splitting in energy. This splitting is affected by: the nature of the metal ion the metal's oxidation state (a higher oxidation state leads to a larger splitting) the arrangement of the ligands around the metal ion th ...
TRANSITION METAL CHEMISTRY –PART 3 –class notes
... Crystal Field Theory is a model that helps explain why some complexes are high spin and some are low spin. Crystal Field Theory views bonding in complexes as the result of electrostatic interactions and considers the effect of ligand charges on energies of metal ion d orbitals. d-electron to ligand- ...
... Crystal Field Theory is a model that helps explain why some complexes are high spin and some are low spin. Crystal Field Theory views bonding in complexes as the result of electrostatic interactions and considers the effect of ligand charges on energies of metal ion d orbitals. d-electron to ligand- ...
learning objective review summary
... Write equations for the reactions of Na2O, MgO, P4O10 and SO2 with water. Explain that some complex ions are coloured because the d-orbitals split and electron transitions take place, absorbing energy from the visible spectrum and causing the complementary colour to be seen (for example, if red ...
... Write equations for the reactions of Na2O, MgO, P4O10 and SO2 with water. Explain that some complex ions are coloured because the d-orbitals split and electron transitions take place, absorbing energy from the visible spectrum and causing the complementary colour to be seen (for example, if red ...
Transition metals and complex ions
... Explain the term ligand in terms of coordinate bonding. Describe and use the terms: complex ion and coordination number. State and give examples of complexes with six-fold coordination with an octahedral shape. ...
... Explain the term ligand in terms of coordinate bonding. Describe and use the terms: complex ion and coordination number. State and give examples of complexes with six-fold coordination with an octahedral shape. ...
RS 10B
... 5) If there is a Jahn Teller distortion that makes the z ligands move out and x & y moving in: how do the d orbitals move? a) dz2 ; dxy ; dx2-y2 ; dxz same; dyz same, ...
... 5) If there is a Jahn Teller distortion that makes the z ligands move out and x & y moving in: how do the d orbitals move? a) dz2 ; dxy ; dx2-y2 ; dxz same; dyz same, ...
Chapter 22-Newest-CD
... Crystal-Field Theory • Crystal field theory describes bonding in transition metal ...
... Crystal-Field Theory • Crystal field theory describes bonding in transition metal ...
Synthesis of hydroborate compounds as potential chemical vapor
... For many transition metal boride phases, it is not possible to use single source CVD precursors to grow films because no precursor of stoichiometry MBxHy exists. Volatile M(BH4)n complexes of d-block transition metals are rare because the BH4- ligand is sterically small and strongly reducing, and in ...
... For many transition metal boride phases, it is not possible to use single source CVD precursors to grow films because no precursor of stoichiometry MBxHy exists. Volatile M(BH4)n complexes of d-block transition metals are rare because the BH4- ligand is sterically small and strongly reducing, and in ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... b) Construct Orgel diagram for d3and d7 systems for tetrahedral and octahedral complexes. (5+5) 25. a) Explain why is the stretching frequency of νC - N shifted to higher ν while that of νC - O is lowered on back donation from metal to ligand? b) For [Cr(H2O)6]2+, the mean pairing energy (P) is 23,5 ...
... b) Construct Orgel diagram for d3and d7 systems for tetrahedral and octahedral complexes. (5+5) 25. a) Explain why is the stretching frequency of νC - N shifted to higher ν while that of νC - O is lowered on back donation from metal to ligand? b) For [Cr(H2O)6]2+, the mean pairing energy (P) is 23,5 ...
Scientific abstract
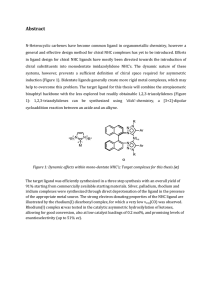
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
... chiral substituents into monodentate imidazolylidene NHC’s. The dynamic nature of these systems, however, prevents a sufficient definition of chiral space required for asymmetric induction (Figure 1). Bidentate ligands generally create more rigid metal complexes, which may help to overcome this prob ...
Crystal Field Theory www.AssignmentPoint.com Crystal Field
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
... dz2 and dx2-y2, which will have higher energy, because the former group is farther from the ligands than the latter and therefore experience less repulsion. The three lower-energy orbitals are collectively referred to as t2g, and the two higher-energy orbitals as eg. (These labels are based on the t ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).