Magnetism of atoms an ions
... mechanics of orbital and spin momenta, their coupling and the rules that govern the occupation of single electron states in isolated atoms or ions in electronic shells. The atomic electron-electron interaction will be discussed leading to Hund’s rules that give the ground state configuration of open ...
... mechanics of orbital and spin momenta, their coupling and the rules that govern the occupation of single electron states in isolated atoms or ions in electronic shells. The atomic electron-electron interaction will be discussed leading to Hund’s rules that give the ground state configuration of open ...
fourth midterm examination
... antibonding MO with a resulting reduction in the t2g-eg (HOMO-LUMO) gap. In addition, with the same ligand, the value of is less with tetrahedral complexes since there are only 4 M-L bonds instead of 6. c) The paramagnetic susceptibility and the value of magnetic dipole moment are strikingly diffe ...
... antibonding MO with a resulting reduction in the t2g-eg (HOMO-LUMO) gap. In addition, with the same ligand, the value of is less with tetrahedral complexes since there are only 4 M-L bonds instead of 6. c) The paramagnetic susceptibility and the value of magnetic dipole moment are strikingly diffe ...
Toward Computational Design of Iron-Based
... role as chromophores in artificial systems for solar energy conversion, such as dye-sensitized solar cells (DSSCs). Fe(II)-polypyridines share many properties with Ru(II)-polypyridines, which have been successfully used as photosensitizers in DSSCs. Visible light excitation in both types of compound ...
... role as chromophores in artificial systems for solar energy conversion, such as dye-sensitized solar cells (DSSCs). Fe(II)-polypyridines share many properties with Ru(II)-polypyridines, which have been successfully used as photosensitizers in DSSCs. Visible light excitation in both types of compound ...
chemistry 1000 - U of L Class Index
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
Lectures 31-33
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
... these atoms come from the same molecule/ion or from several different ones. Go back and assign a co-ordination number to ...
Critical Thinking Worksheet 9
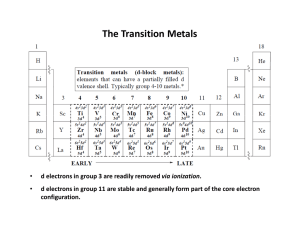
... complexes in the first three rows of the table. If this process leads to unpaired electrons, the complex is paramagnetic and is attracted towards magnetic field. If there are no unpaired electrons, the complex is diamagnetic and is weakly repelled by magnets. ...
... complexes in the first three rows of the table. If this process leads to unpaired electrons, the complex is paramagnetic and is attracted towards magnetic field. If there are no unpaired electrons, the complex is diamagnetic and is weakly repelled by magnets. ...
InorgCh425
... The rates of reaction depend on the ability of the electron to “tunnel” through the ligands from one metal to the other i. Tunneling = moving through an energy barrier (the ligands) that is normally too high to allow the electron to pass through. This is a quantum mechanical process having to do wit ...
... The rates of reaction depend on the ability of the electron to “tunnel” through the ligands from one metal to the other i. Tunneling = moving through an energy barrier (the ligands) that is normally too high to allow the electron to pass through. This is a quantum mechanical process having to do wit ...
Chapter 22 - U of L Class Index
... necessary to look at the absorption of light other than green. Generally, we will try to choose the wavelengths most strongly absorbed by the complex. On the figure above, that would correspond to the peak yellow-red wavelength or the peak violet ...
... necessary to look at the absorption of light other than green. Generally, we will try to choose the wavelengths most strongly absorbed by the complex. On the figure above, that would correspond to the peak yellow-red wavelength or the peak violet ...
15.2 COMPLEX FORMATION And THE SHAPE OF COMPLEX IONS
... some ligands attach themselves using two or more lone pairs classified by the number of lone pairs they use multidentate and bidentate ligands lead to more stable complexes ...
... some ligands attach themselves using two or more lone pairs classified by the number of lone pairs they use multidentate and bidentate ligands lead to more stable complexes ...
Chapter 2 - U of L Class Index
... necessary to look at the absorption of light other than green. Generally, we will try to choose the wavelengths most strongly absorbed by the complex. On the figure above, that would correspond to the peak yellow-red wavelength or the peak violet ...
... necessary to look at the absorption of light other than green. Generally, we will try to choose the wavelengths most strongly absorbed by the complex. On the figure above, that would correspond to the peak yellow-red wavelength or the peak violet ...
Spin crossover

Spin Crossover (SCO), sometimes referred to as spin transition or spin equilibrium behavior, is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to external stimuli such as a variation of temperature, pressure, light irradiation or an influence of a magnetic field.With regard to a ligand field and ligand field theory, the change in spin state is a transition from a low spin (LS) ground state electron configuration to a high spin (HS) ground state electron configuration of the metal’s d atomic orbitals (AOs), or vice versa. The magnitude of the ligand field splitting along with the pairing energy of the complex determines whether it will have a LS or HS electron configuration. A LS state occurs because the ligand field splitting (Δ) is greater than the pairing energy of the complex (which is an unfavorable process).Figure 1 is a simplified illustration of the metal’s d orbital splitting in the presence of an octahedral ligand field. A large splitting between the t2g and eg AOs requires a substantial amount of energy for the electrons to overcome the energy gap (Δ) to comply with Hund’s Rule. Therefore, electrons will fill the lower energy t2g orbitals completely before populating the higher energy eg orbitals. Conversely, a HS state occurs with weaker ligand fields and smaller orbital splitting. In this case the energy required to populate the higher levels is substantially less than the pairing energy and the electrons fill the orbitals according to Hund’s Rule by populating the higher energy orbitals before pairing with electrons in the lower lying orbitals. An example of a metal ion that can exist in either a LS or HS state is Fe3+ in an octahedral ligand field. Depending on the ligands that are coordinated to this complex the Fe3+ can attain a LS or a HS state, as in Figure 1.Spin crossover refers to the transitions between high to low, or low to high, spin states. This phenomenon is commonly observed with some first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves are a common representation of SCO phenomenon with the most commonly observed types depicted in Figure 2 in which γHS (the high-spin molar fraction) is plotted vs. T. The figure shows a gradual spin transition (left), an abrupt transition with hysteresis (middle) and a two-step transition (right). For a transition to be considered gradual, it typically takes place over a large temperature range, even up to several hundred K, whereas for a transition to be considered abrupt, it should take place within 10 K or less.These curves indicate that a spin transition has occurred in a metal complex as temperature changed. The gradual transition curve is an indication that not all metal centers within the complex are undergoing the transition at the same temperature. The abrupt spin change with hysteresis indicates a strong cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of external stimuli (temperature in this case) for the two phenomena, namely LS → HS and HS → LS. The two-step transition is relatively rare but is observed, for example, with dinuclear SCO complexes for which the spin transition in one metal center renders the transition in the second metal center less favorable.There are several types of spin crossover that can occur in a complex; some of them are light induced excited state spin trapping (LIESST), ligand-driven light induced spin change (LD-LISC), and charge transfer induced spin transition (CTIST).