Stereoselective synthesis: chiral auxiliaries
... ....................cleavage conditions must not damage product! ...
... ....................cleavage conditions must not damage product! ...
ALCOHOLS
... replaced by a hydroxyl group. They have the general formula C nH2n+1OH. The functional group (OH) gives alcohols their characteristic properties. The first members of the series are: CH3OH ...
... replaced by a hydroxyl group. They have the general formula C nH2n+1OH. The functional group (OH) gives alcohols their characteristic properties. The first members of the series are: CH3OH ...
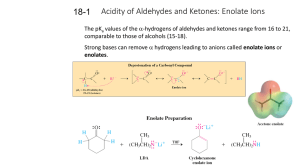
18-1 Enolates (PPT)
... However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more substituted ketone carbonyl. ...
... However, for acetaldehyde, the enol form is about 100-times more stable than that of acetone because the less substituted aldehyde carbonyl is more stable than the more substituted ketone carbonyl. ...
Carboxylic acids Acyl chlorides Amides Esters
... contain a CC triple bond are unsaturated (more H atoms (or other atoms) can be added to their molecules) ...
... contain a CC triple bond are unsaturated (more H atoms (or other atoms) can be added to their molecules) ...
Pyrrolidine-2-carboxylic Acid (l
... Abhimanyu S. Paraskar obtained his Masters degree in chemistry from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
... Abhimanyu S. Paraskar obtained his Masters degree in chemistry from Amravati University, Maharastra, India. He is currently working on his PhD thesis under the supervision of Dr. Arumugum Sudalai at National Chemical Laboratory, Pune, India. ...
Synthesis Explorer
... Aldehydes and ketones can be distinguished by using either Fehling’s solution or Tollens’ reagent. Aldehydes give a red precipitate of copper(I) oxide when warmed with Fehling’s solution, while ketones do not react. Similarly aldehydes produce a silver mirror on the inside of the test tube when warm ...
... Aldehydes and ketones can be distinguished by using either Fehling’s solution or Tollens’ reagent. Aldehydes give a red precipitate of copper(I) oxide when warmed with Fehling’s solution, while ketones do not react. Similarly aldehydes produce a silver mirror on the inside of the test tube when warm ...
Tin-Catalyzed Esterification and Transesterification Reactions: A
... responsible for 65% of the final prices [5]. Alternativefeedstoks have been proposed for their production, such as animal fats, waste frying, and vegetable oil refining processes rejects. Nevertheless, these low-cost raw materials contain high FFA amount and are not compatible with alkaline catalyst ...
... responsible for 65% of the final prices [5]. Alternativefeedstoks have been proposed for their production, such as animal fats, waste frying, and vegetable oil refining processes rejects. Nevertheless, these low-cost raw materials contain high FFA amount and are not compatible with alkaline catalyst ...
File
... 1. Compare addition polymers to condensation polymers. 2. Copy Table 2.1: “Examples of Addition Polymers” on page 83 and Table 2.2: “Examples of Condensation Polymers” on page 84. 3. Describe using structural formula the formation of Nylon-66. 4. Compare polyamides to polyesters. 5. Using your textb ...
... 1. Compare addition polymers to condensation polymers. 2. Copy Table 2.1: “Examples of Addition Polymers” on page 83 and Table 2.2: “Examples of Condensation Polymers” on page 84. 3. Describe using structural formula the formation of Nylon-66. 4. Compare polyamides to polyesters. 5. Using your textb ...
Meeting future SO2 emission challenges with Topsøe`s new
... circumstances for which the catalyst is blown with hot air for a sufficiently long time, up to 10% of the catalyst weight is desorbed as SO2/SO3 leaving the alkali metals in the form of sulfates, M2SO4. The catalytic activity of the melt was demonstrated conclusively in 1948 by Topsøe and Nielsen [3 ...
... circumstances for which the catalyst is blown with hot air for a sufficiently long time, up to 10% of the catalyst weight is desorbed as SO2/SO3 leaving the alkali metals in the form of sulfates, M2SO4. The catalytic activity of the melt was demonstrated conclusively in 1948 by Topsøe and Nielsen [3 ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.